-
PDF
- Split View
-
Views
-
Cite
Cite
Jun-Ho Lee, Tae-Hoon Kim, Hyo Eun Park, Eun Gae Lee, Nam-Chul Jung, Jie-Young Song, Han Geuk Seo, Ki-Bae Seung, Kiyuk Chang, Dae-Seog Lim, Myosin-primed tolerogenic dendritic cells ameliorate experimental autoimmune myocarditis, Cardiovascular Research, Volume 101, Issue 2, 1 February 2014, Pages 203–210, https://doi.org/10.1093/cvr/cvt246
Close - Share Icon Share
Abstract
Autoimmunity plays an important role in the pathogenesis of viral myocarditis and giant cell myocarditis. Experimental autoimmune myocarditis (EAM) is a mouse model of myocarditis that is induced by cardiac myosin. Tolerogenic dendritic cells (tDCs) are used as anti-inflammatory and immunosuppressive targets in a number of autoimmune disease models, but their effect on EAM has not been addressed. The aim of this study was to investigate whether tDC therapy in an EAM mouse model can suppress inflammatory myocarditis, a potential precursor of dilated cardiomyopathy.
tDCs were generated by treating immature DCs (imDCs) with TNF-α and cardiac myosin. Mice with EAM were injected twice with tDCs (with a 1-week interval) at three doses (2 × 105, 1 × 106, or 2 × 106). The severity of myocarditis was histopathologically assessed. The phenotypes of the DC and regulatory T (Treg) cell populations were determined by flow cytometry and the effect of tDCs on autoimmunity-inducing cytokines was examined by ELISA. Myosin-pulsed tDCs displayed lower levels of DC-related surface markers and expressed higher levels of indoleamine 2, 3-dioxygenase (IDO) than mature DCs (mDCs). Histopathological examination revealed that hearts from tDC-treated mice showed markedly reduced myocardial inflammation compared with those of untreated EAM mice. These therapeutic effects by tDCs were mediated at least by enhanced myosin-specific Treg cell induction and anti-inflammatory cytokine secretion.
Taken together, these results show for the first time that myosin-pulsed tDCs ameliorate EAM, and that this occurs most likely via the induction of antigen-specific Treg cells.
1. Introduction
Autoimmune myocarditis occurs as a result of an aberrant interaction between infection and immunity.1,2 Although infective pathogens are major aetiological factors causing this disease, autoimmunity between T-cell and cardiac antigens is a probable major pathogenic mechanism of cardiac injury.3–5 Pathogen-derived antigens activate T-cell migration from the periphery to the heart. On encountering the same antigen in the myocardium, these T cells then undergo considerable dendritic cell (DC)-mediated expansion.6,7 Treatment of inflammatory cardiomyopathy, however, is not fully effective.8–10
Normally, DCs regulate immune homoeostasis by maintaining the balance between T-cell immunity and tolerance.7,11 Among the different DC subsets, tolerogenic dendritic cells (tDCs) play an important role in inducing peripheral tolerance via specific mechanisms, including activation of regulatory T (Treg) cells, suppression of effector T cells, and negative modulation of Th1/Th2 immune responses.12–14 Although the exact mechanisms that induce a tDC to become immunogenic or tolerogenic in vivo have not been elucidated, increasing evidence suggests that tDC function is pivotal for the maintenance of immune homoeostasis. Recently, an intermediate stage of DC maturation was described, characterized by higher levels of MHC class II and co-stimulatory molecule expression than immature DCs (imDCs), but lacking pro-inflammatory cytokine secretion.14,15 This stage of maturation was achieved by exposing imDCs to TNF-α and a specific antigen ex vivo; the cells were termed antigen-specific tDCs. These tDCs induced tolerance by generating Treg cells, which secreted IL-10 but were otherwise uncharacterized.12,14,16,17
Evidence from a number of animal models indicates that specific antigen-loaded tDCs can suppress the onset and/or progression of autoimmune disease; for example, by adoptive transfer of bone marrow-derived DCs pulsed with self-antigens into mice with experimental autoimmune encephalitis,18 autoimmune uveitis,19,20 collagen-induced arthritis,21–23 or type 1 diabetes.24 For these reasons, tDCs are used as anti-inflammatory and immunosuppressive targets in the study of various autoimmune diseases;23,25 however, their effect on autoimmune myocarditis has not yet been addressed.
Experimental autoimmune myocarditis (EAM) is a CD4+ T-cell-mediated disorder involving a Th1/Th2 imbalance, and is induced by immunization with cardiac myosin. Cardiac tissue obtained from EAM mice shows severe myocardial damage and large areas of myocyte necrosis and fibrosis similar to those observed in human myocarditis.26–29
The aim of the present study was to generate tDCs ex vivo by exposing imDCs to TNF-α and cardiac myosin and to investigate their contribution to the regulation of myocarditis in an EAM mouse model.30,31
2. Methods
2.1 Generation of bone marrow-derived DCs
DCs were generated from bone marrow progenitors of 6-week-old male Balb/c mice, as described previously,23,25,27,32 with some modifications. Briefly, bone marrow single-cell suspensions were prepared, the cells were washed, and the washed cells were cultured in RPMI 1640 supplemented with 10% FBS (Life Technologies, Grand Island, NY, USA), 20 ng/mL recombinant mouse GM-CSF (JW CreaGene, Gyeonggi, Korea), and 2 ng/mL rmIL-4 (JW CreaGene) at 37°C with 5% CO2. On Day 3, non-adherent cells were washed and re-fed using the same culture conditions. On Day 6, 50% of the culture medium was replaced with fresh medium containing the same concentrations of GM-CSF and IL-4. After 8 days of culture, tDCs and mature DCs (mDCs) were generated by additional incubation with 10 ng/mL rmTNF-α (BD Biosciences, Mountain View, CA, USA) and 50 µg/mL cardiac myosin (Sigma-Aldrich, St Louis, MO, USA) or 50 µg/mL type II collagen (Sigma-Aldrich) for 4 h, and with 1 µg/mL lipopolysaccharide (Sigma-Aldrich) and 50 µg/mL cardiac myosin for 24 h, respectively. DCs were tested for the expression of CD11c, CD14, CD40, CD54, CD80, CD86, MHC class I, and MHC class II (BD, Pharmingen, San Diego, CA, USA) and for propidium iodide (Sigma-Aldrich) uptake (to measure cell survival), by flow cytometry using a FACSCalibur flow cytometer (BD Biosciences).
2.2 Administration of tDCs to EAM mice
All animal experiments were conducted in accordance with the animal welfare guidelines of CHA University and the Catholic University Guide for the Care and Use of Laboratory Animals. For in vivo experiments, animals were anaesthetized by intraperitoneal (i.p.) injection of 50 mg/kg Zoletil 50 (Virbac Korea, Seoul, Korea). Additionally, for histological studies, immune status evaluation, and the isolation of primary cells, animals were sacrificed via cervical dislocation. Mice were immunized with 100 µg cardiac myosin emulsified 1:1 in complete Freund's adjuvant (Difco Laboratories, Detroit, MI, USA), as described previously.23,31 On Day 7, mice were boosted with the same dose of antigen in incomplete Freund's adjuvant (Difco). On Day 8, EAM-induced mice were injected subcutaneously (s.c.) with 2 × 105, 1 × 106, or 2 × 106 myosin-pulsed tDCs, 1 × 106 type II collagen-pulsed tDCs, 1 × 106 antigen-unpulsed tDCs, or PBS. The injections were repeated 1 week later. Mice were sacrificed on Day 21. Spleens and hearts were collected from each group for in vivo immune status (cytokine production, surface antigen, and mRNA expression) and immunohistochemical analyses.
2.3 Histopathological evaluation
Serial heart sections (5 µm) were cut and every fifth section was stained with haematoxylin and eosin (H&E). The severity of myocarditis was scored histologically from grade 0 to 4 as follows: 0, no inflammatory infiltrate; 1, small foci of inflammatory cells; 2, larger foci of >100 inflammatory cells; 3, >10% of the section involved; 4, >30% of the section involved.33 Immunohistochemical analyses were performed using anti-CD3 (Abcam, Cambridge, MA, USA) and anti-F4/80 (Millipore, Temecula, CA, USA) primary antibodies to detect T cells and macrophages, respectively. The staining was developed using the HistoMouse™-MAX kit (IHC secondary Ab #87-9551) (Invitrogen, Carlsbad, CA, USA).
2.4 DC/T-cell co-culture
Each DC subset (imDC, tDC, or mDC) (2 × 105cells/mL) was co-cultured with CD4+ T cells (isolated using a CD4 MicroBead mouse kit: Miltenyi Biotec, Auburn, CA, USA) (1 × 106 cells/mL) from splenocytes of naive Balb/c mice. The mixed cells were cultured for 72 h at 37°C with 5% CO2 in 2 mL RPMI 1640 supplemented with 10% FBS. For Treg cell analysis by flow cytometry, the mixed cells were stained with anti-CD4, anti-CD25, and then FoxP3-staining buffer (eBioscience, San Diego, CA, USA) according to the manufacturer's instructions.
2.5 Cytokine measurement
The levels of cytokines (IL-23, IL-12p70, IL-10, IL-4, IL-22, IL-17, and IFN-γ) were measured in the culture supernatants from DC alone, from the DC+CD4+ T-cell co-cultures, and from the splenocytes of DC-vaccinated EAM mice, using commercial ELISA kits according to the manufacturer's instructions (eBioscience).
2.6 RNA isolation and real-time PCR
RNA was isolated from each DC subset (imDC, tDC, or mDC), splenocytes, and hearts using TRIZOL Reagent (Life Technologies, Mount Waverley, Australia). Total RNA was reverse transcribed using Superscript II reverse transcriptase (Life Technologies) and random primers (Promega, Madison, WI, USA). cDNA samples were subjected to real-time quantitative PCR (qRT–PCR) analyses with primers and a SYBR Green qPCR kit (Applied Biosystems, Warrington, UK) for FoxP3, indoleamine 2,3-dioxygenase (IDO), and GAPDH, using a Bio-Rad C1000TM thermal cycler. PCR mixtures were cycled for 10 min at 95°C followed by 40 cycles of 15 s at 95°C and 60 s at 60°C. The primers used for real-time PCR were as follows: FoxP3: forward-TCA AAG AGC CCT CAC AAC CAG CTA, reverse-TTT GAA GGT TCC AGT GCT GTT GC; IDO: forward-CAG CTT CTC CTG CAA TCA AAG CA, reverse-TGC GAG GTG GAA CTT TCT CAC AGA; GAPDH: forward-AAC TTT GGC ATT GTG GAA GGG CTC, reverse-TGG AAG AGT GGG AGT TGC TGT TGA. The normalized value for FoxP3 mRNA expression was calculated as the relative quantity of FoxP3 divided by the relative quantity of GAPDH. Samples were run in triplicate.
2.7 Statistical analysis
Statistical analysis was performed using the GraphPad software (GraphPad Prism v5.0; GraphPad Software). Data presented as means ± SD were submitted to one-way ANOVA followed by the Newman–Keuls test. A P-value of <0.05 was considered statistically significant (Supplementary material online, Tables S1–S3) .
3. Results
3.1 tDCs induce further IDO expression compared with mDCs
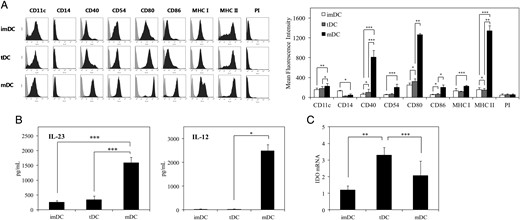
To determine the phenotype of the tDCs generated from mouse bone marrow-derived DCs by treatment with TNF-α and cardiac myosin, the expression of DC-related cell surface markers (CD11c, CD14, CD40, CD54, CD80, CD86, MHC class I, and MHC class II) was analysed by flow cytometry. Compared with mDCs, tDCs showed a lower constitutive expression of co-stimulatory markers and surface MHC molecules, with levels similar to those of imDC (Figure 1A). Using this phenotypic difference to define the two DC subsets (tDC and mDC), the results showed that tDCs produced lower levels of IL-12p70 and IL-23 (Figure 1B), and also higher levels of IDO mRNA (Figure 1C), than the mDC subset.
Characterization of tDC. (A) DC subsets (imDC, tDC, and mDC) were stained with the directly conjugated antibodies indicated and analysed by flow cytometry. Data are shown as histograms (representative of 10 independent DC preparations; each DC preparation was prepared from a different mouse) (left) and as a bar graph of mean fluorescence intensity (right). The bar graphs shown express mean florescence intensity as means ± SD (n = 10 independent DC preparations). (B) DC subsets were cultured in the absence of GM-CSF, IL-4, and TNF-α for 24 h; the supernatant was then harvested and analysed for IL-23 and IL-12 by ELISA. Results are expressed as means ± SD (n = 5 independent DC preparations). Assays for each DC preparation were performed in triplicate. (C) Indoleamine 2,3-dioxygenase (IDO) mRNA levels in DC subsets determined by qRT–PCR. The normalized value for IDO mRNA expression was calculated as the relative quantity of IDO divided by the relative quantity of GAPDH. Results are expressed as means ± SD (n = 5 independent DC preparations). Assays for each DC preparation were performed in triplicate. *P < 0.05; **P < 0.01; ***P < 0.001.
3.2 tDCs induce tolerogenic conditions when co-cultured with T cells
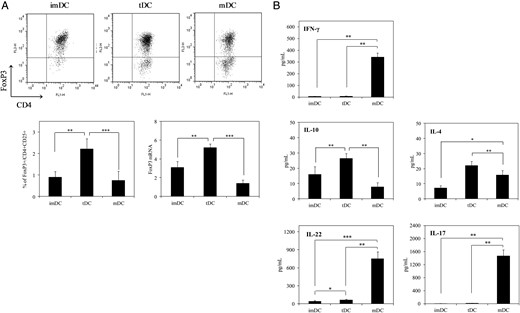
To investigate the influence of tDCs on Treg cell expansion, a series of functional co-culture experiments were performed. As shown in Figure 2A, tDCs markedly increased the FoxP3+ Treg cell population, as measured by both protein and mRNA levels, compared with mDCs and imDCs. Additionally, compared with the mDC subset, myosin-pulsed tDCs inhibited production of IFN-γ and Th17 cytokines (IL-22 and IL-17) (Figure 2B). In contrast, compared with imDCs and mDCs, cultures with tDCs produced more IL-10 and IL-4 (Figure 2B). These results imply that the tDCs have a potential to induce tolerance when co-cultured with T cells.
Immunosuppressive characteristics of tDCs. (A) Each DC subset (2 × 105cells/mL) was co-cultured with CD4+ T cells (1 × 106 cells/mL) from splenocytes of naive Balb/c mice for 72 h. Ten independent tDC+CD4+ T cell co-culture preparations were used for this experiment (each co-culture preparation was prepared from a different mouse). For Treg cell analysis, the mixed cells were stained with anti-CD4, anti-CD25, and then FoxP3 staining buffer and analysed by flow cytometry. The upper right quadrant in the dot plots shows the population of CD4+FoxP3+ cells present after culture with imDC, tDC, or mDC (representative of 10 independent co-culture preparations). The lower left bar graph shows the percentage of CD4+CD25+FoxP3+ cells in the mixed cultures (mean of 10 independent co-culture preparations). FoxP3 mRNA levels were determined by qRT–PCR. The normalized value for FoxP3 mRNA expression was calculated as the relative quantity of FoxP3 divided by the relative quantity of GAPDH. Results are expressed as means ± SD (n = 10 independent co-culture preparations) (assays for each preparation were performed in triplicate). (B) Cytokine levels in the supernatants after 72 h were quantified by ELISA. Results are expressed as means ± SD (n = 5 independent co-culture preparations) (assays for each preparation were performed in triplicate). *P < 0.05; **P < 0.01; ***P < 0.001.
3.3 Vaccination with tDCs prevents progression of acute myocarditis
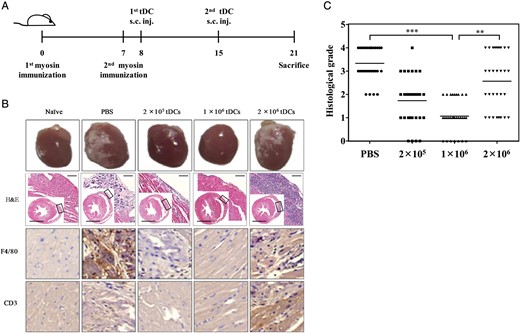
Cardiac myosin is the main component of heart muscle that induces EAM. EAM was induced, using the classical protocol of immunization with cardiac myosin on experimental Days 0 and 7 (Figure 3A). Compared with the hearts of naive mice, animals pre-immunized with myosin showed significantly more wide-spread inflammation of the pericardium (the PBS group in Figure 3B). In addition, F4/80 and CD3 immunostaining identified marked infiltration by inflammatory cells and T-cells between the heart muscle layers (the PBS group in Figure 3B). To determine whether tDCs have a therapeutic effect on established EAM, mice were injected twice (on Days 1 and 7 after EAM onset) with 2 × 105, 1 × 106, or 2 × 106 tDC, or with PBS alone. Mice were sacrificed on Day 21 and the organs examined. The effect of tDCs on the development of myocarditis was analysed by cardiac histology. Hearts were excised for observation of gross findings, and cut sections were microscopically examined after staining with H&E. Repeated experiments demonstrated that the treatment of EAM mice with low doses of tDCs abrogates the onset of EAM (Figure 3B). EAM symptoms were not observed at all in the animals injected with 1 × 106 tDCs, while EAM symptoms were accelerated in the mice vaccinated with 2 × 106 tDCs, and the severity of symptoms was similar to that observed in the untreated control EAM mice. Pathological myocarditis was scored as follows: 0, normal; 1, mild; 2, moderate; 3, marked; and 4, severe. Injection of 2 × 105 or 1 × 106 tDCs after induction of EAM led to a reduction in the myocarditis score compared with that in non-treated mice (PBS group) (Figure 3C). Thus, tDC-mediated tolerization effectively decreased disease incidence, or reduced severity in most of the mice that developed disease. These results imply that tDCs are very potent inhibitors of EAM progression when vaccinated at an appropriate dosage.
Vaccination with low doses of tDCs suppressed EAM development in mice. (A) The vaccination schedule. (B) EAM-induced mice were injected s.c. with 2 × 105, 1 × 106, or 2 × 106 myosin-pulsed tDCs or PBS. Mice were sacrificed on Day 21 and hearts were collected (n = 5 mice for each group, except for untreated naive, n = 1). The hearts of mice from each group were selected randomly and photographed. Serial heart sections (5 µm) were cut and every fifth section was stained with H&E. Immunohistochemical analyses were performed using anti-CD3 and anti-F4/80 primary antibodies to detect T cells and macrophages, respectively. Open squares in the insets are magnified sites and indicate infiltration by inflammatory cells. Long-scale bars: 2000 µm; short-scale bars: 200 µm. Data shown are representative of six independent experimental sets. (C) The severity of myocarditis was scored histologically from grade 0 to 4 (0, no inflammatory infiltrate; 1, small foci of inflammatory cells; 2, larger foci of >100 inflammatory cells; 3, >10% of the section involved; 4, >30% of the section involved). Data shown are expressed as means of 30 mice in each group. **P < 0.01; ***P < 0.001.
3.4 Effect of tDC therapy on Treg cell induction and cytokine production
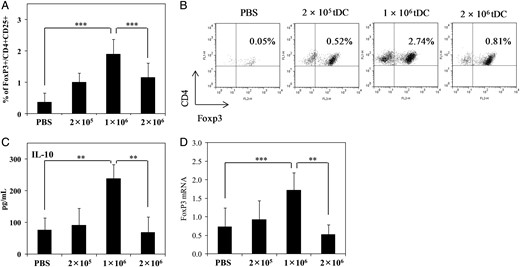
To study the in vivo effects of tDC treatment on the phenotype of the splenic Treg cell population and on cytokine production, splenocytes from tDC-treated mice were harvested 7 days after the second injection of tDCs and compared by flow cytometry with those from non-treated mice. As shown in Figure 4A and B, CD4+CD25+FoxP3+ Treg cells increased markedly in the spleens of mice vaccinated with 1 × 106 tDCs compared with those observed in other groups. Figure 4A and B shows the mean value of five mice in each group and representative FACS dot plots, respectively. At the same time, increased production of IL-10 was observed in the supernatants of splenocytes (cultured with myosin for 72 h) from EAM mice injected with 1 × 106 tDCs compared with other groups (Figure 4C; the mean value of five mice in each group is shown). Additionally, the level of FoxP3 mRNA increased prominently in the hearts of mice vaccinated with 1 × 106 tDCs compared with that observed in other groups (Figure 4D). These results support the histopathological findings shown in Figure 3.
Vaccination with a low dose of tDCs significantly enhanced the Treg population in spleens and hearts of EAM mice. (A) For Treg cell analysis by flow cytometry, splenocytes harvested on Day 21 of the schedule were stained with anti-CD4-, anti-CD25-, and then FoxP3-staining buffer. The percentage of CD4+CD25+FoxP3+ cells are shown. Data are means ± SD of 30 mice in each group. (B) Dot plots showing the population of CD4+FoxP3+ cells (upper right quadrant). Data shown are representative of six independent experimental sets. (C) IL-10 levels in splenocyte supernatants after 72 h of culture were quantified by ELISA. Data shown are the means ± SD of 30 mice in each group (assays for each mouse were performed in triplicate.). (D) FoxP3 mRNA levels from the hearts of EAM mice determined by qRT–PCR. The normalized value for FoxP3 mRNA expression was calculated as the relative quantity of FoxP3 divided by the relative quantity of GAPDH. Data shown are means ± SD of 30 mice in each group (assays for each mouse were performed in triplicate). **P < 0.01; ***P < 0.001.
3.5 Expansion of a myosin-specific Treg cell population
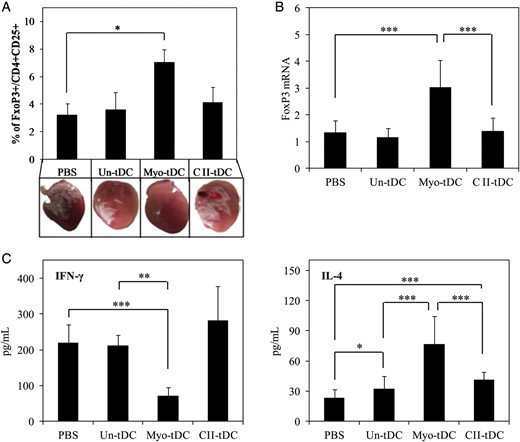
Effective suppression of immune responses in EAM by Treg cells requires that these cells migrate to appropriate sites, respond to antigen, and induce down-regulation of myocarditis’ severity. To confirm the myosin-specific Treg cell expansion in vivo, antigen-unpulsed, antigen-mismatched (type II collagen-pulsed), or myosin-pulsed tDCs were vaccinated in the same way as above. On Day 21, each mouse was sacrificed and splenocytes were harvested. The splenocytes were cultured with myosin (50 µg/mL) for 72 h and the Treg cell population (FoxP3+CD4+CD25+) was evaluated by flow cytometry (Figure 5A). At the same time, FoxP3 mRNA levels in the splenocytes were measured by qRT–PCR (Figure 5B). The Treg cell population, as measured by phenotype and FoxP3 mRNA levels, was markedly increased in the EAM mice that received myosin-pulsed tDC. At the same time, decreased production of IFN-γ was observed in the supernatants of splenocytes (cultured with myosin for 72 h) from EAM mice injected with myosin-pulsed tDCs compared with other groups (Figure 5C). In contrast, increased production of IL-4 was observed in the supernatant of splenocytes from EAM mice that received myosin-pulsed tDC (Figure 5C). These results suggest that myosin-loaded tDCs expanded the population of myosin-specific Treg cells, demonstrating that the Treg cells were antigen specific. Photographs of hearts from mice in each group are shown in Figure 5A.
Myosin-pulsed tDCs induced an antigen-specific Treg cell population. (A) EAM-induced mice were injected s.c. with antigen-unpulsed (Un-tDC), myosin-pulsed (Myo-tDC), or type II collagen-pulsed (CII-tDC) (antigen-mismatched) tDCs or PBS. Six days after the second tDC vaccination, mice were sacrificed and splenocytes harvested. For Treg cell analysis by flow cytometry, the splenocytes were stained with anti-CD4-, anti-CD25- and then FoxP3-staining buffer. The percentage of CD4+CD25+FoxP3+ cells are shown. Data shown are means ± SD of 10 mice in each group. Photographs show the hearts of mice in each group (representative of two independent experimental sets). (B) FoxP3 mRNA levels from the splenocytes were determined by qRT–PCR. The normalized value for FoxP3 mRNA expression was calculated as the relative quantity of FoxP3 divided by the relative quantity of GAPDH. Data shown are means ± SD of 10 mice in each group (assays for each mouse were performed in triplicate). (C) IFN-γ and IL-4 levels in splenocyte supernatants after 72 h of culture were quantified by ELISA. Data shown are the means ± SD of 10 mice in each group (assays for each mouse were performed in triplicate). *P < 0.05; **P < 0.01; ***P < 0.001.
4. Discussion
Autoimmunity is important in the generation of myocarditis.26,34 In particular, a reaction to cardiac myosin following viral infection may contribute to the development of dilated cardiomyopathy, which can be fatal.2,26 The aetiology of myocarditis, however, is unclear and although immunosuppressive drug treatments can be effective in the absence of infective pathogens, a treatment that targets the immune imbalance does not yet exist.8,28,35 In the present study, the use of myosin-pulsed tDCs as a therapeutic tool was examined in an EAM mouse model. The antigen-loaded tDCs generated displayed a more immature phenotype and produced more IDO than mDCs.36 The results of our in vivo studies suggest that tDCs suppress autoreactive T-cell activity and maintain immune homoeostasis.11,17,23
EAM is characterized by severe myocardial damage, including infiltration of mononuclear cells into the myocardium and the appearance of multinucleated giant cells.3,4,37 The cellular infiltrate consists predominantly of T lymphocytes, monocytes, and macrophages, all of which are recruited from the circulation.
EAM is induced by T cell activation, and DCs are powerful antigen-presenting cells that are adept at both stimulating naive T cells and at controlling the subsequent immune responses. The control exercised by DCs is determined by the subset, state of maturation (classifiable as functionally mature or immature), and type and duration of signals received. DCs form a heterogeneous group of cells, which play an important role in both stimulating immunogenicity and in maintaining immune tolerance. To promote tolerance, DCs induce antigen-specific T-cell responses that result in anergy, apoptotic deletion, or Treg cell formation. In this context, they are referred to as tDCs and the majority show poor immunostimulatory capacity, express IDO, and may induce FoxP3+ Tregs rather than activate effector T lymphocytes. Adaptive effector T-cell responses to self- and non-self-antigens can be efficiently controlled by Tregs belonging to the CD4+CD25+ subset. Tregs are phenotypically heterogeneous and include both CD4+ and CD8+ T cells, most of which express FoxP3. Failure of Treg function is implicated in the development of a variety of autoimmune processes and, vice versa, cellular therapies involving the adoptive transfer of Tregs are effective against these disorders. In addition, DCs regulate Treg homoeostasis and vice versa. IL-10 is unique among cytokines because it can induce FoxP3 expression and Treg differentiation in the absence of DC.23,38
To advance the field of DCs as a therapeutic tool for the treatment of autoimmune myocarditis, methods for the generation and delivery of tolerogenic or vaccinated DCs for EAM must first be standardized, and efficacy tests in the chronic phase of the disease should be also performed. The results of the present study show that cardiac myosin-pulsed tDCs have a clear therapeutic effect on EAM, which is associated with acute myocarditis, a model of an organ-specific autoimmune disease. Injecting 2 × 105 or 1 × 106 tDCs after the onset of disease significantly reduced the accumulation of pathogenic immune cells in heart muscle, whereas treatment with higher numbers of tDCs (2 × 106) exacerbated inflammation markedly. These results imply that tDCs are very potent inhibitors of EAM progression when vaccinated at an appropriate dosage. The positive effects of tDCs required loading with myosin, a heart-specific antigen, which suggests that tDCs modulated the immune response in an antigen-specific manner (Figure 5). Compared with non-tDC-treated groups, tDC promoted the expansion of FoxP3+ Treg cells. tDC therapy also markedly increased the levels of FoxP3 mRNA expression in the hearts of mice vaccinated with 1 × 106 tDCs, implying that negative immune modulation-promoting Treg cells contribute to the suppression of EAM (Figure 4D).
Our finding that tDCs at high densities are immunogenic would be explained as follows; tDCs in the semi-mature stage might be further maturated via vivid DC–DC interactions at high densities in the presence of T cells after injection, thereby resulting in the adoption of immunogenic, rather than tolerogenic qualities. It has been established that DCs acquire antigens shed from other cells and, particularly, from other DCs. However, we could not detect any surface phenotype changes of tDCs even in the high density of tDCs before co-culturing with CD4+ T cells or inoculation into EAM mice. The capacity of DCs both to shed antigens and to acquire shed antigens allows for effective antigen transfer between DCs, thereby suggesting that high densities of tDCs may possibly stimulate each other, followed by the induction of immunity rather than tolerance in the immune system environment.
In conclusion, this study clearly shows that tDCs pulsed with cardiac myosin effectively ameliorate myocardial inflammation in a mouse model of EAM by inducing myosin-specific Treg cells. These results suggest that tDCs hold promise as a novel therapeutic strategy for myocarditis.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: none declared.
Funding
This study was supported by a grant of the Korean Health Technology R&D project, Ministry for Health and Welfare, Republic of Korea (A092258).
References
Author notes
J.-H.L. and T.-H.K. contributed equally to this study.
- cytokine
- myocarditis
- phenotype
- tumor necrosis factors
- immunosuppressive agents
- enzyme-linked immunosorbent assay
- myosins
- flow cytometry
- antigens
- autoimmunity
- cardiac myosins
- dendritic cells
- t-lymphocytes
- heart
- mice
- regulatory t-lymphocytes
- transcranial direct current stimulation
- episodic ataxia type 1