-
PDF
- Split View
-
Views
-
Cite
Cite
Signe Carlson, JoAnn Trial, Christian Soeller, Mark L. Entman, Cardiac mesenchymal stem cells contribute to scar formation after myocardial infarction, Cardiovascular Research, Volume 91, Issue 1, 1 July 2011, Pages 99–107, https://doi.org/10.1093/cvr/cvr061
Close - Share Icon Share
Abstract
Therapeutic advances in prevention and treatment of myocardial infarction (MI) have decreased patient mortality and increased concern about efficient repair and scar formation, processes that are necessary to attenuate complications such as adverse remodelling and heart failure. Since the rapid accumulation and activity of cardiac fibroblasts is critical for proper scar formation, we hypothesized that infarct fibroblasts are generated by a cardiac-resident progenitor cell population.
We found that infarct fibroblasts in C57BL/6 mice are generated by a mesenchymal stem cell (MSC) population that responds robustly to injury by proliferating and accumulating in the infarct. We report that stem cell-derived fibroblasts contribute to the formation of a scar after an infarction by differentiating into matrix-producing fibroblasts closely associated with fibrillar collagen in the infarct. Further characterization of these cells revealed a heterogenous population with expression of both stem cell and canonical cardiac fibroblast markers, suggesting that some have a commitment to the fibroblast phenotype. Our in vitro study of these cells shows that they have extended self-renewal capability and express the primitive marker Nanog. In keeping with these observations, we also report that these cells are multipotent and differentiate readily into fibroblasts as well as other mesenchymal lineages.
Cells with the properties of MSCs participate in wound healing after MI in the adult heart.
1. Introduction
With the increased survival rate from myocardial infarction (MI) over the last two decades, the primary pathophysiological concern has shifted to cardiac repair and effective scar formation.1 Evidence suggests that these are important factors in morbidity and mortality and are the major variables in adverse remodelling post-infarction.2,3
Our laboratory has examined repair after MI in large mammals and in mice.4 Repair of murine MI begins with acute inflammation and debridement of the infarcted area, followed by matrix deposition and scar formation mediated by fibroblasts.4,5 These studies demonstrated the existence of a mechanism that generates a large population of fibroblasts within 72 h after injury. We hypothesized that endogenous mesenchymal stem cells (MSCs) contribute to the infarct fibroblast population.
Our study demonstrates that the fibroblasts forming in the scar of an MI arise from an endogenous pool of primitive CD44+ mesenchymal precursors that localize and proliferate in the wound within 36 h, as shown by expression of the cell cycle marker Ki67. This population of CD44+ cells lack the haematopoietic marker CD45 as well as the inflammatory markers CD18 and CD73, indicating that they are not bone marrow-derived. Furthermore, we demonstrate that a portion of these cells are multipotential stem cells that express the stem cell markers CD34, TERT, and Nanog and are capable of differentiating into multiple lineages in vitro as well as into CD44+ fibroblasts such as those seen in the infarct. In contrast, structural fibroblasts in the heart are CD44-negative and lineage restricted. Recent studies by others have suggested the importance of MSC therapy in cardiac repair.6–8 These studies support the role of endogenous MSCs as important mediators of repair by generating and processing the extracellular matrix of the forming scar.
2. Methods
2.1 Animals
Twelve- to 16-week-old male C57BL/6 mice were obtained from the Center for Comparative Medicine at Baylor College of Medicine. All mice were fed standard mouse chow and water ad libitum. The investigation conformed with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health. All animals were treated in accordance with the guidelines of the Baylor College of Medicine Animal Care and Use Committee.
Mice were anaesthetized and implanted with a reversible occluder as described,9 then allowed to recover for 1 week prior to undergoing 1h of coronary occlusion followed by various periods of reperfusion. Sham-operated animals were implanted but not occluded.
2.2 Cell isolation
Mice were anaesthetized by isoflurane and sacrificed by cervical dislocation. The whole hearts were immediately processed to obtain a single cell suspension of the non-myocyte cells (see Supplementary material online). Cell suspensions were used directly for immunofluorescence staining and cell culture or enriched for CD44.
Cells to be enriched for CD44 positivity were incubated with anti-CD44 coupled to ferrous microbeads and applied to an autoMACS cell sorter (Miltenyi Biotec, Auburn, CA, USA). Purity was confirmed at >95% (see Supplementary material online).
2.3 Immunofluorescence and flow cytometry
Cells were incubated with antibodies to cell surface markers or isotype controls. 50 nmol/L calceinAM (which forms a fluorescent green product only in metabolically active cells) was added to some samples to define living cells. Some samples were fixed before incubation with antibodies to internal antigens or IgG controls followed by the appropriate secondary antibody when indicated (see Supplementary material online). Cells were analysed on a Cell Lab Quanta SC flow cytometer (Beckman Coulter) using the Quanta Analysis software. The histograms shown were generated with FlowJo software (Tree Star, Inc., Ashland, OR, USA).
2.4 Fluorescence microscopy
Infarcted hearts were fixed with 1.5% zinc as published.10 Standard methods were used for paraffin embedding, sectioning, and immunofluorescence staining.11 To dim autofluorescence, stained slides were incubated with 0.3% Sudan Black B12 (see Supplementary material online). Immunofluorescence microscopy was performed on an Olympus AX70 microscope. Images were taken on a black and white digital camera with QCapture Pro software, then assigned false colour and merged using ImageJ software (NIH).
2.5 Two-photon microscopy and second harmonic generation imaging
Hearts were treated as above until dehydrated to 70% ethanol, then cryopreserved, frozen, and sectioned to 40 μm. Sections were mounted on glass slides, air dried, and stained as above except the Sudan Black step was omitted.
Tissue slides were imaged on the stage of a modified Zeiss LSM 410 confocal microscope.13 Illumination for two-photon and second harmonic generation (SHG) imaging was provided by a mode-locked titanium sapphire laser (Coherent Mira 900). See Supplementary material online for more details. Strong SHG signals in the sample were generally recorded from collagen14 and myosin15 filaments. The fluorescence signals from Alexa-488- and Alexa-594-labelled secondary antibodies in these tissue slides were also excited by the 800 nm illumination and the two-photon excited emission was collected simultaneously with the SHG signals by the LSM 410 using appropriate emission filters.
2.6 Cell culture
Cells were grown on tissue culture-treated plastic in DMEM:F12 (1:1) with 10% foetal bovine serum (FBS, HyClone, Logan, UT, USA). Some cultures were enriched for primitive cells by progressively lowering media glucose and serum concentrations prior to culture in stem cell medium (SCM) designed to inhibit differentiation (see Supplementary material online, Figure S1). Reagent sources are described in Supplementary material online.
Cells for differentiation into fibroblasts were passaged from SCM directly into fibroblast differentiation medium (FDM) consisting of DMEM:F12/10% FBS with 10 ng/mL TGF-β1 (R&D Systems) on plastic until the cells reached confluency 3–4 days later. Phase-contrast images were taken on an Olympus CKX41 inverted microscope with QCapture Pro software before the cells were detached for flow cytometry.
Cells for osteocytic, chondrocytic, and adipocytic differentiation were plated overnight in SCM, then rinsed with D-PBS and changed to differentiation medium as described16 or the appropriate control medium, and fed every 2 days for 2–3 weeks, then fixed and stained with Alizarin Red S, pH 8.2 (osteocytes), Alcian Blue, pH 2.5 (chondrocytes),17 or Oil Red O (adipocytes) according to the published methods18 (see Supplementary material online).
2.7 Statistical analysis
All data are expressed as mean ± SE. One-way ANOVA was used to evaluate differences between groups and post hoc testing was done using the Tukey–Kramer method. A P-value of <0.05 was considered statistically significant and is indicated by an asterisk in the figures.
3. Results
3.1 CD44+ cells that were not of bone marrow origin (CD45neg) accumulated in the infarct shortly after MI
Analysis of cells from infarcted hearts showed the presence of a cell population that expressed CD44 but not CD45 (Figure 1C and D), indicating that they are not bone marrow-derived. Similarly, the CD44+CD45neg population lacked the inflammatory markers CD18 and CD73 (data not shown). Flow cytometry analysis (Figure 1C) showed that the number of CD44+CD45neg cells increased by 20% at 2 days and by >100% at 3 days post-infarction over sham-operated animals, remained elevated through 5 days, and decreased to sham levels by 7 days. To further support our hypothesis that these cells indicated a resident population, we also infarcted mice with a genetic deletion of monocyte chemoattractant protein 1. Our previous work has shown that these mice are defective in attracting bone marrow-derived cells to the infarcted heart; however, their scar formation is not compromised.19 Consistent with those results, we observed that although CD45+ cell recruitment to the heart was restricted, CD44+CD45neg cells increased as in the wild-type mice (see Supplementary material online, Figure S2), suggesting that their increase is not due to recruitment from the bone marrow. The timing of CD44+CD45neg cell accumulation and decline coincides with that of fibroblasts and myofibroblasts in the infarct as reported in our previous work.4
CD44+ cells proliferated in the infarct. (A) Representative flow cytometry histogram showing viable (calcein+) non-myocytes with CD44 and CD45. (B) Section from the sham-injured heart showing CD44+CD45neg cells (red). (C) Cytometry data showing CD44+CD45neg cells as a per cent of viable non-myocytes 2–7 days after infarction. (D) Sections from a 2-day infarct showed CD44+ (red) and CD45+ (green) cells in the infarct. (E) Flow cytometry showed that the number of proliferating (Ki67+) CD44+ cells peaked at 3 days after infarction. (F) A section from a 2-day infarct showed proliferating (Ki67+, green) CD44+ cells (red) within the infarct. Nuclei stained with DAPI (blue). Scale bar = 20 μm. Data shown as mean ± SE; *P < 0.05 [n = 8, 10, 6, 6, and 6 for sham through 7 days in (C) and n = 8, 6, 6, 6, and 6 for sham through 7 days in (E)]
To further examine the increase in cell number, we analysed the expression of Ki67, a nuclear marker of proliferative cells. Flow cytometry showed a significant rise in the number of CD44+CD45negKi67+ cells after infarction, with a 2.5-fold increase over sham-operated mice (Figure 1E). Indeed, more than 70% of the CD44+CD45neg cells were proliferating at 2-day post-infarction. Proliferation stayed elevated through 5 days and decreased nearly to sham levels by 7 days. Inspection of sections by microscopy confirmed our cytometry data and showed CD44+ cells with nuclear expression of Ki67 within the infarct by 2 days (Figure 1F).
3.2 CD44+CD45neg cells became fibroblasts and myofibroblasts in the infarct
Quantitative analysis of CD44+ cells from infarcted hearts indicated that the cells had internal stores of collagen type I (Figure 2A) and expressed the canonical cardiac fibroblast marker, DDR2 (Figure 2B), both peaking in expression at day 3. However, as befits a sequential maturation of progenitors to fibroblasts and then myofibroblasts, the expression of α-smooth muscle actin (α-SMA) was not manifest until later in the time course (Figure 2C).
CD44+ cells became fibroblasts and myofibroblasts after MI. Cytometry data showing CD44+CD45neg cells after infarction with (A) collagen type I, (B) DDR2 and (C) α-SMA. (D) Trichrome staining of 3-day infarction. At this time, (E) CD44+ cells (green) and collagen type I (red) were present in the infarct. Few collagen fibrils were seen (blue) and (F) few myofibroblasts (α-SMA+, green) were present. Arrowheads show CD44+α-SMA+ cells. (G) Trichrome staining of 5-day infarction. At this time, (H) collagen type I (red) was more organized and fibrils (blue) were closely associated with CD44+ cells (green). (I) Expression of α-SMA (green) in the infarct substantially increased, indicating myofibroblast accumulation with more CD44+ (red) α-SMA+ (green) double-positive cells present. Scale bar = 20 μm. Data shown as mean ± SE; *P < 0.05 [n = 8, 4, 6, 5, and 7 for sham through 7 days in (A) and n = 7, 4, 5, 6, and 4 for sham through 7 days in (C)].
Matrix must be cross-linked and contracted as well as deposited for successful scar formation. To study the time course of these processes in association with the CD44+ cells, we combined the use of SHG with immunofluorescence. The newly deposited collagen type I in the infarcted area was identified by immunofluorescence, but this technique did not discern its fibrillar structure. The physical structure of collagen can be determined by SHG, a phenomenon wherein photons that interact with a fibrillar molecule are combined to yield new photons that emit at half the wavelength originally focused on the sample. Combining SHG with two-photon immunofluorescence imaging allowed us to visualize both newly deposited non-fibrillar collagen type I and cross-linked fibrillar collagen within the infarct. SHG also revealed the sarcolemmal structure of cardiomyocytes adjacent to the infarcted area, which were easily distinguished from the collagen matrix by their characteristic sarcomeric pattern.
Inspection of 3-day infarcts (Figure 2D, E, and F) showed that collagen type I was deposited in the infarct around CD44+ cells but that there were few fibrils at this time, indicating that the scar was still immature (Figure 2E). Consistent with the cytometric data, there were few α-SMA+ cells in the infarct (Figure 2F).
In contrast, 5-day infarcts (Figure 2G, H, and I) showed abundant collagen type I deposited in the infarct and organized fibrils that were closely associated with CD44+ cells (Figure 2H). Myofibroblasts (α-SMA+) were also clearly seen throughout the infarct at 5 days (Figure 2I).
3.3 CD44+ cells freshly isolated from uninjured hearts expressed stem cell and fibroblast markers
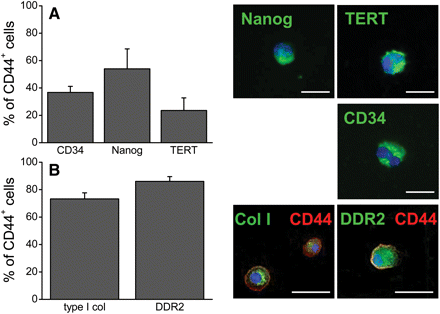
To further characterize the CD44+ population, we examined cells from uninjured hearts. Flow cytometry analysis showed that CD44+ cells were a heterogeneous population, with varying expression of the stem cell markers CD34 (37%), Nanog (54%), and telomerase reverse transcriptase (TERT, 24%; Figure 3A). It has been reported that CD34 participates in the asymmetric division of progenitor cells,20 Nanog indicates multipotentiality,21 and TERT is well known to maintain telomere length. Expression of these markers indicated a progenitor cell population within the heterogeneous CD44+ population. Immunofluorescence microscopy confirmed our cytometry data and allowed us to assess the cellular localization of the antigens, which was both nuclear and cytoplasmic for Nanog and TERT (Figure 3A).
CD44+ cells from the uninjured heart expressed stem cell and fibroblast markers. (A) Uninjured hearts were analysed for CD44 and the stem cell markers CD34, Nanog, and TERT by cytometry to determine the percentage of CD44+ cells with primitive markers (n = 4). Microscopy shown for confirmation. (B) In addition, a majority of CD44+ cells (red) from uninjured hearts contained collagen type I and expressed DDR2 (n= 8). Microscopy showed small amounts of collagen type I in a perinuclear location as well as DDR2 expression (both in green). Nuclei stained with DAPI (blue). Data shown as mean ± SE. Scale bar = 20 μm.
In addition, we found that a majority of CD44+ cells expressed fibroblast markers. Flow cytometry showed that 73% of the CD44+ cells contained collagen type I and 86% expressed DDR2 (Figure 3B). These cells appeared small, with little cytoplasm, and the collagen type I was sequestered in organelles. These data suggest that some CD44+ progenitor cells may be biased towards the fibroblast lineage even in uninjured tissue.
3.4 Heterogeneous population of CD44+ cells from infarcted hearts were enriched for primitive cells in vitro
Owing to the low number of CD44+ cells in the uninjured heart, we opted to isolate CD44+ cells from 3-day infarcts, when they are rapidly dividing but their identity as stem cells is unknown. The non-myocyte cells from several hearts were pooled to increase yield and immunomagnetically separated for CD44+ cells, and then cultured in medium with 10% FBS to encourage cell division and to expand the culture.
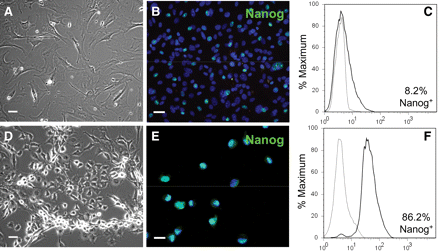
Our observations of the cellular morphology of these cultures (Figure 4A), their expression of fibroblast markers DDR2 (90.1 ± 3%) and collagen type I (92.6 ± 2%) as well as vimentin (>85%), and their lack of endothelial (Tie-1, CD31), smooth muscle (SM1, desmin), and pericyte (NG2, RGS5) markers indicated that the cultures were proliferating primarily as fibroblasts; however, the cultures were not contact inhibited and surpassed the Hayflick limit (>25 passages). This suggested a heterogenous culture in which the fibroblasts were being replenished by a pool of primitive cells with extended self-renewal capability. To test this hypothesis, we evaluated the expression of Nanog. We found that 8% of the cultured cells were positive at passages 7–11 for Nanog (Figure 4C) by flow cytometry, with microscopy confirming expression in the nucleus (Figure 4B). This confirmed the presence of mesenchymal precursors and supported our model of culture heterogeneity in which a few stem cells give rise to a majority population of maturing fibroblasts.
CD44+ cells were enriched for Nanog in SCM. CD44+ cells isolated from infarcted hearts and cultured in medium with 10% FBS maintained Nanog in <10% of the cells: (A) phase-contrast microscopy, (B) fluorescence microscopy, and (C) flow cytometry of anti-Nanog immunofluorescence (black line) compared with isotype control (grey line). When cultured in a serum-free SCM, the cells were smaller (D) and Nanog+ cells comprised almost 90% of the culture (E and F) (n = 2). Scale bar = 20 μm.
To better evaluate the primitive CD44+ population separately from the differentiated fibroblasts in our cultures, we developed a selection method to discourage fibroblast growth and enrich for the Nanog+ cells in vitro (see Supplementary material online, Figure S1). This method caused the death of differentiated cells and produced more homogenous cultures consisting primarily of small, rounded cells (Figure 4D), of which nearly 90% showed nuclear expression of Nanog (Figure 4 and F). Expression of the MSC marker CD90 was variable in the cultured cells with no apparent relationship between expression and differentiation capability. In addition, 90–98% of the cultured cells expressed collagen type I and DDR2 throughout the enrichment process, suggesting a lineage bias towards the fibroblast phenotype even while maintaining multipotentiality. At no time did the cultures express CD31, desmin, Tie-1, NG2, RGS5, or SM1; however, vimentin, collagen type I, and DDR2 were expressed throughout.
3.5 CD44+ progenitor cells from infarcted hearts were multipotential and differentiated into other mesenchymal lineages
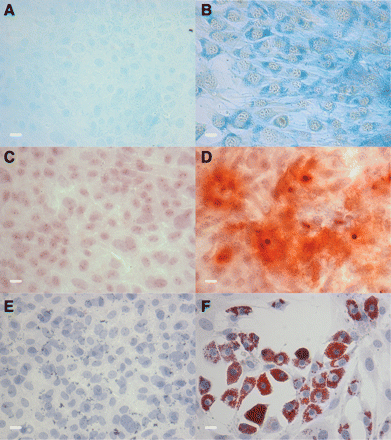
Nanog expression has been well established as an indicator of multipotentiality in progenitor cells.22 Therefore, we cultured the putative stem cells to induce various mesenchymal lineages (Figures 5 and 6) and observed differentiation into chondrocytes (Figure 5B), osteocytes (Figure 5D), and adipocytes (Figure 5F). Cells cultured in an equivalent basal medium lacking induction factors failed to differentiate into these lineages (Figure 5A, C, and E, respectively), as did fibroblasts from standard cultures (data not shown).
Primitive CD44+ cells were multipotential and differentiated into mesenchymal lineages. Cells cultured in SCM were plated in either control medium (A, C, and E) or medium to induce differentiation to adipocytes (B), osteocytes (D), or chondrocytes (F). Differentiation was confirmed by positive staining for (A and B) Alcian Blue for chondrocytes, (C and D) Alizarin Red S for osteocytes, and (E and F) Oil Red O for adipocytes. Scale bar = 20 μm. Representative images of five experiments.
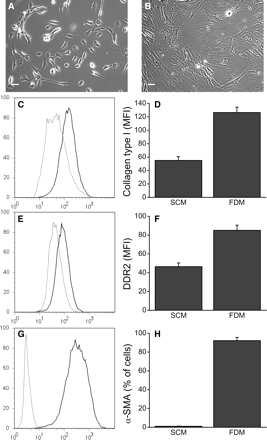
CD44+ cells up-regulated fibroblast markers and could be differentiated into myofibroblasts. Cells cultured in SCM were plated in FDM and analysed by flow cytometry. Phase-contrast images of cells in (A) SCM and (B) FDM. Note the visible stress fibres and typical fibroblast morphology. (C) Cells expressed collagen type I, with SCM showing 94.6 ± 1.1% positive (grey line) and FDM showing 98.6 ± 0.1% positive (black line). (D) The geometric MFI of collagen type I was up-regulated in FDM cultures (55–126). (n = 6). (E) Similarly, cells expressed DDR2, with 98.2 ± 0.26% of SCM (grey line) and 98.9 ± 0.12% of FDM cultures (black line) and (F) the MFI of DDR2 was up-regulated in FDM (46–85) (n = 5). (G and H) SCM cultures (grey line) were only 1% positive for α-SMA, whereas FDM (black line) induced 92% of the cells to express α-SMA, indicating myofibroblast differentiation (n = 6). Data shown as mean ± SE. Scale bar = 20 μm.
3.6 CD44+ progenitor cells differentiated into fibroblasts and myofibroblasts in vitro
We had noted earlier that CD44+ cultures grown in DMEM:F12 with 10% FBS spontaneously differentiated into fibroblasts (Figure 4); however, we did not establish if this was due to the culture medium or influence from differentiated fibroblasts in the cultures. Therefore, we cultured cells enriched for Nanog in SCM in FDM containing TGF-β1 (FDM; Figure 6) until the cells reached confluency (3–4 days). We observed dramatic changes in cellular morphology with this treatment, with cells in FDM increasing in size by 52.9 ± 5.5% (mean channel volume by flow cytometry), adopting fibroblast morphology, and developing visible stress fibres (Figure 6B). Flow cytometry analysis showed that although there was little difference between the SCM and FDM cultures with respect to the total number of cells that make collagen type I (95 and 99% positive, respectively; Figure 6C), there was significant up-regulation of cytoplasmic collagen type I in the FDM cultures (Figure 6D) as measured by the geometric mean fluorescence intensity (MFI). We found similar results with DDR2 expression: 98% of the SCM cultures and 99% of the FDM cultures expressed DDR2 (Figure 6E), but the expression level increased nearly two-fold with FDM treatment (Figure 6F).
Expression of the myofibroblast marker α-SMA in the SCM cultures was very low, with only 1% positive (Figure 6G and H). However, we observed substantial induction of α-SMA (92% positive) with FDM treatment, indicating robust myofibroblast differentiation.
4. Discussion
One of the most important pathophysiological variables in the outcome of an acute MI is the development of an efficient scar that allows mechanical stabilization of the ventricular wall.4 This process arises from complex interactions that initiate inflammation,23 suppress inflammation,24 provide the provisional matrix,5 and mature the scar.4,5 The relationship between inflammation, fibrosis, and healing presents a significant challenge as investigators attempt to limit inappropriate cardiac fibrosis, such as that generated by chronic inflammation, without compromising healing capability in the event of acute myocardial injury.
Wound healing in most organs is accomplished by fibroblasts and myofibroblasts. These cells make up a phenotypic and maturational continuum from the secretory fibroblast that deposits extracellular matrix in the wound to the myofibroblast that contracts the scar. In this paper, we propose that resident CD44+ MSCs provided a major precursor pool for the generation of the fibroblasts that mediate scar formation after infarction.
MSCs are non-haematopoietic stromal cells that give rise to mesenchymal tissues, are self-renewing, and migrate to sites of tissue injury.25 They are identified by their expression of mesenchymal markers, lack of haematopoietic markers, adherence to plastic, and ability to differentiate into osteocytes, adipocytes, and chondrocytes.26 Because of their ability to mature into cells of many lineages (multipotentiality), MSCs have been intensively studied for their contribution to tissue healing and regeneration.7 For example, bone marrow-derived MSCs have been shown in some studies to benefit the healing infarct primarily through paracrine mechanisms.27,28
We characterized fibroblasts in the post-MI heart with respect to their derivation from a primitive mesenchymal precursor and their phenotype in vivo and in vitro. To study the precursors, we relied on the cell surface marker CD44, which is stably expressed by MSCs even in vitro.29 CD44 has been implicated in control of inflammation and scar formation and is a ligand for hyaluronic acid fragments.30 We followed the expansion of CD44+ non-haematopoietic cells in an MI in vivo and ex vivo and their association with collagen deposition in the developing scar. The time points for this study correspond to the chronology that we previously established for infarct healing in the mouse.4,5
The data demonstrate that MSCs exist in the uninjured adult heart in low numbers and that these cells respond to injury by rapidly proliferating and participating in wound healing after MI by generating fibroblasts and myofibroblasts. When the CD44+ precursor cell population is freshly isolated out of the heart, the majority are small cells with limited cytoplasm that express stem cell markers such as Nanog and CD34, whereas structural fibroblasts are CD44-negative and morphologically distinct. In addition, a significant population also contains DDR2, a canonical marker of the cardiac fibroblast31 and cytoplasmic collagen type I, which appears to be localized in organelles (Figure 3). Thus, it appears that this population is heterogeneous and may contain precursor cells at various states of differentiation even in the uninjured heart. The coexistence of primitive and fibroblast markers may represent an intrinsic lineage bias for cardiac MSCs to generate fibroblasts in the event of an injury.
The CD44+ precursor cell population generated fibroblasts in vitro when placed in an appropriate medium (Figure 4). The fibroblast population that is generated is unique and distinct from that of structural fibroblasts in vivo in that they are CD44+, retain primitive markers, and are capable of serial culture significantly beyond the theoretical Hayflick number. This led us to suggest that the CD44+ cells seen at any one time in the mouse heart might present a continuum from primitive MSCs to newly generated fibroblasts. To further investigate this, we developed an enrichment technique to preferentially grow primitive MSCs and characterize them (Figure 4; see Supplementary material online, Figure S1), using Nanog as the criterion for stemness. Nanog is a pluripotent cell-specific gene that maintains the undifferentiated state of stem cells or reprogrammed somatic cells,21 and its expression is indicative of a primitive state. Nanog is also expressed by MSCs found in adult organs, including the heart.32 The enrichment technique allowed us to confirm the multipotentiality of endogenous MSCs in the absence of the larger fibroblast cell population.
We demonstrated that even in the actively proliferative phase of an MI, a population of endogenous MSCs exists as a potential source of additional fibroblasts. The sharp increase in the proliferation of the CD44+ precursor population contributed to the rapid rise in fibroblast number during the first 3 days of the MI (Figure 1). We were able to confirm these observations with our in vitro experiments, in which we found that isolated CD44+ cells have extended self-renewal capability and continually generate fibroblasts under standard growth conditions.
The data demonstrate that cells with the properties of MSCs play a major role in wound healing by generating fibroblasts and myofibroblasts in the adult heart. MSCs in adult tissues have been described with varying degrees of potentiality.33 This study shows that the primitive cells residing in the mouse heart have the ability to differentiate into fibroblasts and myofibroblasts as well as other lineages. Our findings are supported by a reported comparison of gene expression profiles between MSCs and fibroblasts, indicating a relationship between these cell types, with fibroblasts being more differentiated and with more restricted differentiation potential.34 It has been suggested that fibroblasts are the end-stage lineage of a self-renewing multipotential MSC.35 In the heart, these cells function as a robust reserve of fibroblast potential in an organ in which rapid and efficient wound healing is critical to survival.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was supported by the National Institutes of Health (NIH RO1-HL089792 to M.L.E.) and the National Science Foundation (NSF OISE-0714392 to S.C.)
Acknowledgements
We thank Katarzyna Cieslik for helpful discussion and data sharing and Isuru D. Jayasinghe for technical assistance.
Conflict of interest: none declared.



![CD44+ cells proliferated in the infarct. (A) Representative flow cytometry histogram showing viable (calcein+) non-myocytes with CD44 and CD45. (B) Section from the sham-injured heart showing CD44+CD45neg cells (red). (C) Cytometry data showing CD44+CD45neg cells as a per cent of viable non-myocytes 2–7 days after infarction. (D) Sections from a 2-day infarct showed CD44+ (red) and CD45+ (green) cells in the infarct. (E) Flow cytometry showed that the number of proliferating (Ki67+) CD44+ cells peaked at 3 days after infarction. (F) A section from a 2-day infarct showed proliferating (Ki67+, green) CD44+ cells (red) within the infarct. Nuclei stained with DAPI (blue). Scale bar = 20 μm. Data shown as mean ± SE; *P < 0.05 [n = 8, 10, 6, 6, and 6 for sham through 7 days in (C) and n = 8, 6, 6, 6, and 6 for sham through 7 days in (E)]](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/cardiovascres/91/1/10.1093_cvr_cvr061/2/m_cvr06101.gif?Expires=1716427442&Signature=x7vF7yn~-8VF11DS40Ro86JIXJrp9b9KD4t3QZdR4goaqJWnIr95IPNGfT4PJD9GZrE0NNYk8vMbU-qufbrPSzpEHJ2bbvmcQkJeOYxeudD5l~ce3Klk1~jwKYLVICa-xtDPgy9ch0a9cVrYyRZ1jnsMAcbAJVCk3vkd8X05lLf8-J5jasUqAy92LrQCyb71Yx-Lwk-DWuTaRm7kul6enNyjKmv-~yBU-40B-dvPKAAVVfZS02goFgm9WShnNSk4LndmE8eVFNCfXwOaIqlajeLYIY0F3tMaKnt5r~PBYGvwThcyUuYrtH0VXfQGim72VZ2SyYWuUNDn4nDIsJdrxg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![CD44+ cells became fibroblasts and myofibroblasts after MI. Cytometry data showing CD44+CD45neg cells after infarction with (A) collagen type I, (B) DDR2 and (C) α-SMA. (D) Trichrome staining of 3-day infarction. At this time, (E) CD44+ cells (green) and collagen type I (red) were present in the infarct. Few collagen fibrils were seen (blue) and (F) few myofibroblasts (α-SMA+, green) were present. Arrowheads show CD44+α-SMA+ cells. (G) Trichrome staining of 5-day infarction. At this time, (H) collagen type I (red) was more organized and fibrils (blue) were closely associated with CD44+ cells (green). (I) Expression of α-SMA (green) in the infarct substantially increased, indicating myofibroblast accumulation with more CD44+ (red) α-SMA+ (green) double-positive cells present. Scale bar = 20 μm. Data shown as mean ± SE; *P < 0.05 [n = 8, 4, 6, 5, and 7 for sham through 7 days in (A) and n = 7, 4, 5, 6, and 4 for sham through 7 days in (C)].](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/cardiovascres/91/1/10.1093_cvr_cvr061/2/m_cvr06102.gif?Expires=1716427442&Signature=ErW~2HxYEIiN2kd7d2oBWCAFtg9YvUD9m8S0p1jKTJ~de4myxHZKwb3GV4vsxvg5uOgVAP28UGWvJP7j21bNhi8R5TLLgEyzBOD9tm9MeRaT2WhanrEGC5Q0Ik3QWnp1k66ZwOzm2ul2-VA9ESEl3nDn~z5x5wUCTAaCpXiRQn7I4iiLUIVJ8B4vpsrON8MSYmhsXgG5omAWxX3yKPDBYRmjHJVzMbT8W3Rz1cvAJ663AqvB2o8aTzOKDn8A5U8a4PrjsZeIVa7eR~LKBu36L-Se6WJ5RGyTFPZJYSj8bJI5xN6XGeRJ5l4dN4Y4aDe9bltLnC-LhiroO8aPtr0ASw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)