-
PDF
- Split View
-
Views
-
Cite
Cite
Mark Gilchrist, Angela C. Shore, Nigel Benjamin, Inorganic nitrate and nitrite and control of blood pressure, Cardiovascular Research, Volume 89, Issue 3, 15 February 2011, Pages 492–498, https://doi.org/10.1093/cvr/cvq309
Close - Share Icon Share
Abstract
Continual nitric oxide (NO) synthesis is important in the regulation of vascular tone and thus blood pressure. Whereas classically NO is provided by the enzymatic oxidation of l-arginine via endothelial NO synthase, it is now clear that NO can also be generated in mammals from the reduction of nitrite and nitrate. Thus inorganic nitrate derived either from NO oxidation or from dietary sources may be an important storage form of reactive nitrogen oxides which can be reduced back to nitrite and NO when physiologically required or in pathological conditions. The very short half-life of NO and the ready availability of stored nitrite and nitrate make for a very sensitive and responsive blood pressure control system. This review will examine processes by which these storage forms are produced and how augmentation of dietary nitrate intake may have a beneficial effect on blood pressure and other vascular function in humans.
1. Introduction
Soon after the discovery of the l-arginine/nitric oxide (NO) pathway in 1987, it became clear that continual endothelial NO synthesis is important in controlling vascular tone and hence blood pressure in humans.1,2 Vallance et al.3 showed that infusion of l-N monomethyl arginine (LNMMA), an inhibitor of nitric oxide synthase (NOS), when infused into the human forearm causes a doubling of forearm vascular resistance. The surprising but clear conclusion4 from this study was that NO is continually being synthesized and released from arterial vascular endothelium, as LNMMA has no intrinsic vasoconstrictor activity. It was clear, however, that NO itself is short-lived, as it is rapidly oxidized to nitrite in aqueous solutions,4 and directly oxidized to nitrate in the presence of superoxide or oxyhaemoglobin. We now know that inorganic nitrate synthesis in humans (first proved by Mitchell in 1916)5 results (probably entirely) from NO oxidation and may be an important storage form of reactive nitrogen oxides, which can be reduced back to nitrite and then NO under certain physiological and pathological conditions. Importantly, this endogenous source of inorganic nitrate is supplemented in varying amounts by dietary intake of inorganic nitrate.
2. Nitrate/nitrite/NO cycling in humans
Under normal physiological conditions, nitrite, along with NO and nitrate, is part of a complex biological cycle in mammals. There are several pathways thought to be involved in the modulation of the cycling of nitrate, nitrite, and NO. These include oxidation or reduction by bacteria,6–8 haemoglobin and other heme proteins,9–13 xanthine oxidoreductase (XOR),14–18 NOS,19,20 hypoxia,9,21 acid catalysed,22–25 and indeed UV radiation.26 The relative contributions of each will vary considerably from the normal physiological state through times of severe cellular stress.
2.1 Dietary sources of nitrate
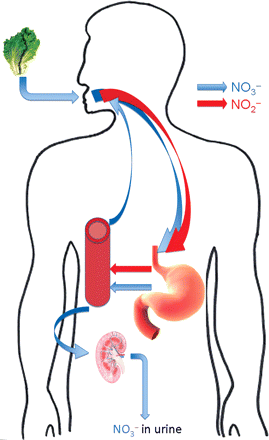
In the typical western diet, an individual will consume 1–2 mmols of nitrate per day27,28 which will rapidly and completely be absorbed in the upper gastrointestinal tract;29,30 60% of ingested nitrate is excreted in the urine within 48 h,31 with the fate of the remainder as yet unknown. The half-life of an oral dose of inorganic nitrate is surprisingly long, estimates ranging between 5 and 8 h.24,31 This is mainly because, although it readily passes through the glomerulus into the renal tubule, it is mostly reabsorbed by the proximal renal tubules. The urinary clearance of nitrate is only about 26 mL/min,31 consistent with the observation that the fractional reabsorption of nitrate by renal tubules is about 90% in dogs,32 by a transport mechanism which has not yet been characterized (Figure 1).
Simplified representation of the entero-salivary circulation of nitrate. Nitrate (represented by the blue arrows) derived from the diet is swallowed. It is rapidly and completely absorbed in the upper gastrointestinal tract. Approximately 25% is concentrated in the salivary glands and secreted into the mouth. Here it is reduced to nitrite (represented by the red arrows) by facultative anaerobes on the dorsum of the tongue and swallowed. Some of the nitrite undergoes acidic reduction to NO in the stomach, with the remainder being absorbed. The fate of nitrite is discussed in depth later. Sixty per cent of ingested nitrate is lost in the urine within 48 h.
2.2 Endogenous production of nitrate
NO from the classical l-arginine NOS pathway is rapidly oxidized to nitrate in the presence of oxyhaemoglobin, which yields approximately 1 mmol nitrate per day.33,34 A by-product of this is methaemoglobin. In this oxidized state, haemoglobin is unable to bind oxygen and requires reduction back to functional haemoglobin by methaemoglobin reductase in order to function again.
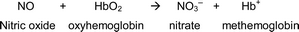
2.3 Entero-salivary circulation of nitrate
Between ingestion or endogenous production and ultimate excretion, circulating nitrate, from whichever source, undergoes complex biological processing in the nitrate/nitrite/NO cycle. From circulating plasma, it is concentrated in the salivary glands and subsequently secreted into the mouth. Approximately 25% of circulating plasma nitrate will be taken up by the salivary glands so that salivary nitrate concentration is at least 10 times that of plasma. On the dorsum of the tongue facultative anaerobes, especially Vionella spp, which can use nitrate instead of oxygen as an alternative electron acceptor, reduce nitrate to nitrite.7 The resulting salivary nitrite concentration is more than 1000 times greater than that of plasma in the resting state.35 This nitrite is then swallowed. In the acidic conditions of the stomach, there is good evidence that nitrite can be reduced to NO, a reaction that occurs spontaneously in acid solutions.24 This intragastric NO production appears to play an important role in regulating gastric blood flow and mucus production36–38 as well as host defence against enteric pathogens.22,39
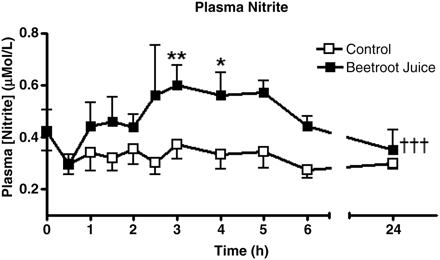
Evidence that some of the nitrite formed in the mouth is also absorbed into the circulation is provided by studies which show an increase in blood nitrite concentration following oral nitrate loading35,40 (Figure 2).
The effect of oral inorganic nitrate (from beetroot juice, administered at time 0) on plasma nitrite. Peak plasma nitrite concentrations occur 3 h following ingestion of nitrate.40
In addition to this pathway for nitrate reduction by tongue anaerobes, Bernheim and Dixon41 had demonstrated in 1928 that both rat muscle and ox liver could reduce nitrate to nitrite. In liver, this was thought in part due to XOR. This capability of mammals was largely overlooked until Lundberg and Weitzberg's group recently provided evidence for a functional mammalian nitrate reductase enzyme demonstrating elevated levels of nitrite following nitrate administration in germ-free and NOS knockout mice.42 The increased nitrite levels were associated with a significant fall in mean arterial pressure (MAP). The rise in nitrite observed was attenuated by allopurinol, suggesting a role for XOR (having excluded NOS and bacteria as potential sources).
2.4 The fate of nitrite
A dose of nitrite administered to experimental animals rapidly disappears. A study in which nitrite was administered to rabbits showed a rapid reduction in plasma concentration from nearly 3000 nm to <1000 nm in <5 min, interpreted as a redistribution phase, but then a slower elimination phase with a half-life of 34 min.43 Kelm44 has shown that in humans the metabolism seems to be more linear with a half-life of 110 s. It was previously thought that reaction with oxyhaemoglobin resulted exclusively in nitrate, but this complex reaction has recently been more carefully studied, and it seems that nitrite can also be further reduced to NO by reaction with haemoglobin,45 which may have physiological importance.46
In plasma, in vitro, nitrite also has a short half-life because it is oxidized to nitrate. It is not yet clear what enzymatic or chemical process is involved in this oxidation reaction. Kim and Lancaster47 demonstrated that the catalysis of the oxidation of nitrite to nitrate in rat hepatocytes and hepatocyte extracts involves a mechanism which uses tetrahydrobiopterin. This would represent a means by which a cell can terminate the reversible reaction between NO and nitrite, thus limiting their activity.
Bryan and Feelisch's group provided some of the most revealing insights into the pharmacology of nitrite,48,49 initially demonstrating that concentrations of nitrite were much higher in vascular tissue than in plasma. Intraperitoneal nitrite injection in rats resulted in rapid absorption from the abdominal cavity with widespread distribution, steady state being achieved after approximately 5 min. A dose–response relationship was evident for the S-nitrosation and heme nitrosylation in all (plasma, red blood cell, heart, liver, kidney, lung) apart from two (brain and aorta) tissues studied. Oxidation to nitrate occurred rather more slowly and indeed a significant increase in nitrate levels was only observed following the highest nitrite dose. In contrast the administration of nitrate intraperitoneally did not produce the S-nitrosation or heme nitrosylation in the time period studied. Interestingly, under the physiological conditions studied there was no detectable formation of NO suggesting nitrite may have a direct signalling effect independent of NO. This may be due to post-translational protein modification by protein nitrosation. It is also possible that nitrite itself exerts a direct blood pressure lowering effect.
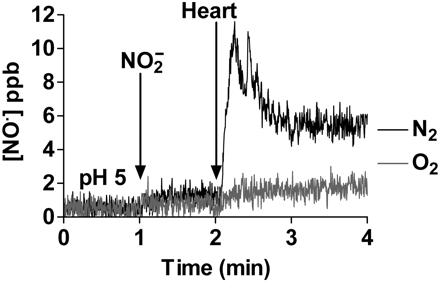
XOR has previously been discussed as a potential nitrate reductase. However, it is more conventionally thought of as a nitrite reductase. In tissues and tissue extracts, nitrite generates much more NO than would be expected at acidic pH, suggesting the presence of a catalyst (Figure 3). Nitrite added to the perfusate in a Langendorff heart preparation results in the formation of gaseous NO which is increased following ischaemia.15 The enzymatic mechanism by which nitrite reduction to NO is enhanced is likely to vary in different tissues. There is good evidence that in the rodent heart XOR is involved. Its activity in red blood cells and vascular endothelial cells under normal physiological conditions appears to be minimal and probably does not contribute in any meaningful way to the regulation of resting vascular tone.20 However, with falling pH and hypoxia there appears to be an increase in the nitrite reductase activity of XOR which makes it more important in the setting of ischaemia–reperfusion injury.15–17,20,50
The addition of rat heart homogenate enhances the release of NO from acidified nitrite.15
3. Effects of inorganic nitrate and nitrite on blood pressure
The vasodilatory properties of organic nitrates and nitrites have been known and put to therapeutic use since the latter part of the nineteenth century, with amyl nitrite being used in the treatment of angina,51 and later nitroglycerin which was used by Alfred Nobel. The vasodilatory effect was short-lived, thus organic nitrate and nitrite therapy was limited to ‘paroxysmal' conditions. Attention would turn to inorganic nitrite compounds in the search for a longer lasting effect.52,53 Reichert's52 paper On the Physiological Action of Potassium Nitrite reviewed the action of potassium nitrite in a host of biological systems in both human and animal models. One surprising finding was that the administration of potassium nitrite salts would cause a transient rise in blood pressure before a blood pressure lowering effect supervened. Attempts to ascertain which nitrite-containing moieties produced the best therapeutic profile while minimizing potential adverse events continued into the early part of last century.54 One agent that appeared to offer a more controlled, predictable, and sustained hypotensive effect was bismuth subnitrate, which Stieglitz noted required reduction by Bacillus coli to nitrous acid allowing slow release of nitrite ions.55,56 This deduction, that bacterial reduction was a required step before nitrate could have a hypotensive effect, had been made some years earlier following investigations into a series of episodes of methaemoglobinaemia in infants who had been administered bismuth subnitrate as a radiological contrast agent.6 While the period of the turn of the last century was marked by a surge in awareness of the potential for nitrite to produce vasodilatation, concerns about methaemoglobinaemia and later nitrite's role as a putative agent in the development in neoplasia57 reduced interest in the vasodilatory component of nitrites pharmacological action. It is perhaps only in the last 20 years or so that nitrite's key role in normal physiological control of blood pressure among other beneficial effects has attracted renewed attention.
3.1 Nitrite and blood pressure
Early investigations had suggested that supra-physiological levels of nitrite had no vasodilatory effect.58 Subsequently the administration of nitrite has been shown to produce vasodilatation in numerous physiologically distinct vascular beds including pulmonary,59–61 cerebral,62–64 and other vascular beds.9,40 The cerebral circulation is worthy of specific consideration when considering the vasodilatory properties and therapeutic potential of nitrite given its autoregulatory system which is key to maintaining cerebral perfusion within desired parameters despite fluctuations in systemic blood pressure. Rifkind and colleagues during a 10 min nitrite infusion showed a biphasic fall in MAP with a falling cerebrovascular resistance which approximately mirrors the fall in MAP.64 Cerebral blood flow, however, is unaffected by the first drop in MAP. Following the second fall in MAP, cerebral blood flow begins to increase. Other studies have shown prevention of vasospasm in a primate subarachnoid haemorrhage model63 or increases in cerebral blood flow in cerebral ischaemia62 following nitrite infusion without any effect on systemic blood pressure. These provide further evidence of nitrite's capacity to produce vasodilatation where vasoconstriction may otherwise supervene and cause harm.
In the pulmonary circulation, whether nebulized61 or administered intravenously59,60 sodium nitrite vasodilates the pulmonary circulation. This attenuated pulmonary hypertension whether induced by hypoxia or infusion of U46619. Interestingly, in Ingram et al.60 study, the pulmonary vasodilation produced by nitrite infusion in man persisted for up to an hour after the nitrite infusion ceased and plasma nitrite had returned to baseline levels.
Deoxyhaemoglobin can reduce nitrite to NO65 resulting in the arterial–venous gradient of plasma nitrite noted by Gladwin's et al.66 This reduction of nitrite, in concentrations found under normal physiological conditions, to NO by deoxyhaemoglobin results in vasodilatation and increased blood flow in the human forearm.9 Considering that metabolism of plasma nitrite increases with falling oxygen tension, Maher et al.21 postulated that this effect would be greatest in the venous circulation under resting conditions. They found that under normoxic conditions low-dose nitrite infusions produced venodilation with no apparent effect on the arterial circulation. As the nitrite dose increased, arterial dilation and an increase in forearm blood flow (FBF) became apparent. Under hypoxic conditions, there was little change to the degree of venodilation. In contrast FBF significantly increased. In the resting state, up to 70% of the circulating blood volume may be in the venous capacitance vessels. Thus, small changes in venous tone may have dramatic effects on systemic blood pressure by decreasing right atrial and ventricular end-diastolic pressure. Venous dilatation and concomitant arterial constriction following administration of nitrite has previously been observed in a study looking into the mechanism behind nitrite-induced circulatory collapse in 1937.67
A significant obstacle to nitrite's vasodilatory activity under aerobic conditions, such as would be expected in the arterial circulation under normal physiological conditions, is the very efficient NO scavenging effect of oxyhaemoglobin.68
Zweier's group showed nitrite reduction to NO and subsequent vasodilatation was mediated by sGC or other heme proteins in the vessel wall.12 Reduction of nitrite by effectors in the vessel wall is an attractive pathway for two reasons: first, this mechanism does not rely on the escape of NO from the red blood cell; and secondly, the concentration of nitrite in vascular tissue is far greater than in plasma.49 Indeed, plasma levels under normal physiological conditions are insufficient to produce arterial dilatation.
Cao and colleagues suggest that nitrite has an indirect effect in promoting vascular NO synthesis. They demonstrated that under hypoxic conditions nitrite promotes ATP synthesis and release by erythrocytes.69 The ATP released under these conditions stimulates endothelial nitric oxide synthase (eNOS) in the vessel wall to produce NO70 with vasodilatation. Nitroglycerin has been reported to have a similar but more modest effect on red blood cell ATP release.71
Interestingly, during Bryan and Feelish's pharmacokinetic study,48 a rise in blood pressure was observed when low doses of nitrite were administered. Other investigators have noted a similar rise in blood pressure when lower doses of nitrite are administered or at the start of an infusion.52,72 Vasoconstriction by sodium nitrite has also been demonstrated in an isolated dog liver model by Geumei et al.73 Bryan and Feelish suggest the rise in blood pressure they observed may represent a feedback mechanism whereby increasing concentrations of nitrite initially inhibit eNOS production of NO before a threshold is reached and a vasorelaxant effect supervenes.
3.2 Evidence that nitrate may be the important factor in high vegetable diet and blood pressure reduction
Decreased production of NO is evident in essential hypertension and other conditions associated with elevated blood pressure such as diabetes, hypercholesterolemia, and chronic kidney disease.33,74–78
A diet rich in fruit and vegetables has been shown to be effective in reducing blood pressure.79,80 In addition, it lowers the risk of morbidity and mortality from cardiovascular disease81–83 as diet exhibits such tremendous intra- and inter-individual variation, elucidating which components of such a diet are responsible for this effect is difficult. There is a growing weight of evidence from both human and animal studies that nitrate and nitrite derived from the diet can serve as a source for NO, particularly where it is deficient.84 Indeed, the greatest protective effect on cardiovascular disease is to be found in those diets with the greatest consumption of green leafy and cruciferous vegetables,82,83 which typically have high nitrate contents.85
Using an L-NAME-induced hypertensive rat model, Tsuchiya et al.86,87 demonstrated the ability of dietary nitrite-derived NO to attenuate the ensuing hypertension and ameliorate the renal injury in rats treated with L-NAME for 8 weeks.
Larsen et al.88 showed a significant fall in diastolic and mean arterial blood pressure associated with a rise in plasma nitrite following the administration of oral sodium nitrate to healthy volunteers. Webb et al.,40 using beetroot juice as a source of inorganic nitrate, showed a significant reduction in blood pressure with a maximum effect at 3 h with a strong inverse correlation with plasma nitrite levels. That reduction of nitrate by oral commensal bacteria to nitrite is required to procure a blood pressure lowering effect was demonstrated by the abolition of the rise in plasma nitrite and associated fall in blood pressure if no saliva was swallowed in the 3 h following an oral nitrate load. The administration of an antibacterial mouthwash has a similar effect.89,90 These effects lend strong support to the hypothesis that it is the nitrate content of a diet rich in fruit and vegetables which confers a significant proportion of the cardiovascular benefits of such a diet.80,91
Ahluwalia's group recently demonstrated comparable blood pressure lowering effects between nitrate doses whether administered with beetroot juice or KNO3 capsules.84 These effects were also not apparent when a KCl control was administered. Interestingly, they also demonstrated significant elevations in cGMP concentrations in association with elevated plasma nitrite. NO's effects are known to be mediated by the activation of cGMP.92 This finding provides further evidence to suggest nitrite's effects are mediated via conversion to NO.
3.3 Sunlight, nitrite, and blood pressure
The significant concentrations of nitrate in sweat from skin stores from which NO is generated following reduction by skin commensals to nitrite93 which are thought to play a role in host defence94 may have another purpose. A perhaps overlooked aspect of the regulation of blood pressure is the role of UVA radiation, which may partly explain seasonal variations in blood pressure noted by some investigators.95 The first suggestion this might be so is from in vitro work by Matsunaga and Furchgott96 who demonstrated light-mediated relaxation of rabbit aorta in the presence of inorganic nitrite. Oplander et al.26 showed that whole body UVA irradiation resulted in a maximum fall in MAP of 11.9±1.8% from baseline 15 min after UVA exposure with a residual effect up to 1h later. This was accompanied by significant increases in FBF and flow-mediated vasodilatation. Like the complex chemistry observed in the stomach, the reactions taking place in the dermis are complex with numerous potential intermediates and putative effectors. NO gas is liberated from the skin following UV irradiation. There were significant rises in plasma nitroso compound (RX-NO) species in plasma which highly correlated with the fall in blood pressure and a significant rise in plasma nitrite which did not. In addition, there was a significant increase in intracutaneous S-nitrosothiols. Previous work by Mowbray et al.97 suggests that nitrate is the likely storage form of NO in skin, with nitrite as the photo-reactive intermediate with concentrations of S-nitrosothiols being comparatively far lower and thought to be of less significance.
3.4 Risk/benefit of increasing nitrate intake to reduce blood pressure
Although nitrates and nitrites have been used medicinally and for curing meat for many centuries (reviewed in a recent paper by Butler and Feelisch98), acute toxic effects have only been encountered at very high doses. The toxicity reported has been principally methaemoglobinaemia, but only when several grams of nitrate salt are administered.99 It has been suggested that some early studies may have shown methaemoglobinaemia with lower doses of nitrate due to contamination with nitrite. Nitrite causes acute toxicity in much smaller doses. The LD 50 of inorganic nitrite in laboratory animals is about 2.6 mmol/kg. If extrapolated to man this equates to a dose of 12 g. In fact is likely to be lower at about 4 g100 with significant methaemoglobinaemia occurring with as little as 1 g (14 mmol).
While the benefits of a diet rich in vegetables, and therefore by extension rich in nitrate, in terms of effect on blood pressure and reduced cardiovascular risk appear clear at a population level, it is prudent to remember that any substance with a pharmacological effect will produce undesirable effects in some. Concern emerged in the 1970s as a result of Tannenbaum and Spiegelhalder's work on the potential of dietary nitrate to form N-nitrosamines.101,102
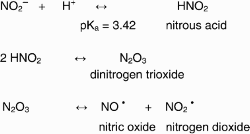
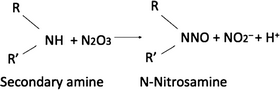
Both nitrous acid and dinitrogen trioxide which are formed from acidification of nitrite are well-known nitrosating agents.103 Nitrosation of secondary amines present in foodstuffs or drugs104 could lead to the formation of carcinogenic N-nitrosamines (see below) which are known, in animal studies, to predispose to gastric and liver cancers.105
It should be noted, however, that there are problems in extrapolating toxicology studies from these small laboratory animals to man. While generally true for most chemical entities, it is especially relevant in any discussion about potential health risks of nitrate. For example, rats do not appear to have the same mechanisms as humans to concentrate nitrate, iodide, and thiocyanate in salivary glands, a feature of nitrate metabolism in man which is key to any potential benefits or harms.106,107
It had been shown by Magee in 1956 that N-nitrosamines could cause hepatic tumours in rats by forming DNA adducts.108 Numerous studies since have attempted to find a positive correlation between nitrate intake or exposure and cancer. However, most are epidemiologically weak and inconclusive.109 Indeed in 2003, the Joint FAO/WHO Expert Committee on Food Additives (JECFA) concluded: ‘Overall, the epidemiological studies showed no consistently increased risk for cancer with increasing consumption of nitrate. These data, combined with the results of the epidemiological studies considered by the Committee at its 44th meeting, do not provide evidence that nitrate is carcinogenic to humans'.110 Furthermore, it has been suggested that it would be surprising from an evolutionary point of view that nitrate would be recovered from glomerular filtrate and actively excreted into the mouth at high concentration if it were a toxic molecule.35
It remains possible that certain subgroups of the human population may be at increased risk of cancer if subject to high levels of dietary nitrate. Winter et al.'s111 paper elegantly demonstrated the formation of nitrosamines in an in vivo system in the human oesophagus. While the ability of a given nitrosamine to induce cancer has considerable inter-species and inter-tissue variation within a species, this is a potentially important finding. Human oesophageal cells can metabolize and activate nitrosamines via the cytochrome p450 enzyme pathway resulting in binding of these metabolites with DNA.112–114 Alcohol, the excessive consumption of which is known to be a risk factor for upper gastrointestinal cancer, inhibits first pass metabolism of nitrosamines by the liver resulting in increased exposure of extra-hepatic organs.115 Thus, for individuals with gastro-oesophageal reflux, the generation of potential carcinogens within the oesophageal lumen in addition to increased circulating nitrosamines may put them at increased risk. It seems reasonable to postulate that those who have subsequently developed dysplasia will be at greater risk still.
Outwith these specific circumstances, nitrite and NO generated in the gastric lumen have a clear role in gastro-protection by increasing gastric blood flow and gastric mucous production.36–38 It also inhibits ulcer formation whether by NSAIDs or in association with H-pylori infection.116,117
4. Conclusion
Nitrate and nitrite play a key role in modulating blood pressure in both health and disease states. The vast array of surprisingly diverse mechanisms by which NO can be generated from nitrite serve to underline its vital role in regulating vascular tone. Its physiological handling appears to be designed to deliver NO to the point of greatest need with evidence that nitrite reduction increases with increasing hypoxia and falling pH—an effect perhaps most evident in certain disease states. In addition, evidence is emerging that nitrite may have a direct vasoactive effect independent of its role as a precursor for NO.
Conflict of interest: N.B. is a cofounder of Heartbeet Ltd, a non-profit making organization set up to promote the health benefits of dietary nitrate.
References
Author notes
This article is part of the Review Focus on: Inorganic Nitrite and Nitrate in Cardiovascular Health and Disease