-
PDF
- Split View
-
Views
-
Cite
Cite
Jurgen W.G.E. VanTeeffelen, Judith Brands, Hans Vink, Agonist-induced impairment of glycocalyx exclusion properties: contribution to coronary effects of adenosine, Cardiovascular Research, Volume 87, Issue 2, 15 July 2010, Pages 311–319, https://doi.org/10.1093/cvr/cvq114
Close - Share Icon Share
Abstract
The endothelial glycocalyx is the negatively charged, gel-like mesh residing at the luminal side of the vascular endothelium and forming the interface between the flowing blood and the vessel wall. The vast majority of glycocalyx volume resides in the microcirculation, particularly in the capillaries. Intravital microscopic observations of capillaries in striated muscle preparations illustrate that under resting conditions, the glycocalyx is not accessible for flowing red blood cells and greatly hinders plasma flow in the axial direction, causing a significant reduction in functionally perfused capillary volume. Glycocalyx exclusion properties have been shown to be reduced by adenosine and other vasoactive substances. A diminished exclusion of circulating blood by the glycocalyx may facilitate nutrient exchange since it is associated with an increase in functionally perfused blood volume and surface area in the capillaries. Our recent studies have focused on the effect of adenosine on glycocalyx exclusion in the coronary circulation and demonstrate an important role for this mechanism in the increase in circulating coronary blood volume during administration of this vasodilator. The current review elaborates on the glycocalyx as a blood-excluding intravascular layer and how it can be modulated by various agonists. Further, the potential role of adenosine-induced modulation of glycocalyx exclusion properties in coupling increases in blood flow and circulating blood volume in the coronary circulation is discussed. Finally, we consider how degradation of the glycocalyx may impact on coronary blood volume regulation, thereby providing new opportunities to diagnose glycocalyx damage in the clinical setting.
1. The endothelial glycocalyx: a carbohydrate-rich layer which contributes to a low capillary tube haematocrit
The idea that the vascular endothelium is lined with a layer of membrane-bound molecules associated with adsorbed proteins was introduced already 75 years ago.1 Later, Copley2 indicated that this compartment could be separated into a thin fibrin-coated layer directly attached to the endothelial membrane and a much larger immobile plasma region next to it. In the meanwhile, electron microscopic studies had evidenced that polysaccharides were abundantly present on the endothelial surface,3 resulting in the term endothelial glycocalyx, or simply glycocalyx, to describe the layer. Although in previous literature, a distinction has been made between the glycocalyx as representing the—relatively thin—layer of membrane-bound macromolecules while referring to the endothelial surface layer as being the sum of this membrane-bound layer and an additional—relatively thick—layer of adsorbed plasma components,4–6 the distinction between the two is regularly neglected these days, and the term glycocalyx is applied to encompass both the endothelial cell (EC) membrane-anchored structures and -adsorbed components. This will also be the case in the current review.
The structural composition of the endothelial glycocalyx has comprehensively been reviewed by others.5–8 The scaffold of the glycocalyx is formed by anionic polysaccharide structures produced by the EC (Figure 1). Heparan sulfate proteoglycans contain abundant binding sites for plasma proteins by specific patterns of sulfation and are able to function as signal transduction molecules via their core proteins, the syndecans and glypicans. The glycosaminoglycan hyaluronan is not sulfated and lacks binding sites for proteins; yet, due to its possible length of several micrometres and its excellent hydration properties,9 it is considered to contribute significantly to the volume of the endothelial glycocalyx.10,11 In the in vivo setting, blood-borne plasma proteins, endothelial-derived growth factors and enzymes, and water are all adsorbed within the polysaccharide mesh, and the glycocalyx seems to appear as a gel-like voluminous compartment at the luminal side of the endothelium. Since capillary endothelial surface area accounts for most of the total surface area of the vascular system, the majority of the glycocalyx volume is indicated to reside in this microvascular compartment.
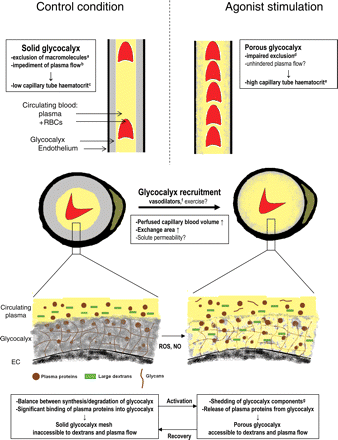
Cartoons illustrating the concept of agonist-stimulated glycocalyx modulation as means of increasing functionally perfused blood volume in the capillaries. Top and middle panels: longitudinal (top) and cross (middle) sections of two capillaries depicting proposed relations between glycocalyx exclusion of flowing RBCs/plasma (yellow) and capillary tube haematocrit/vascular volume available for perfusion, in control conditions (left), and during agonist stimulation (right). Under control conditions, a solid glycocalyx (grey), which excludes circulating blood and macromolecules (a: refs 10,23,24,33,60; b: refs 47,48) lines the endothelium (black), causing capillary tube haematocrit to be low (represented by the low number of RBCs in the left section; c: refs 16,17,19,23,26,33). During stimulation with vasoactive substances, the glycocalyx becomes accessible for large dextrans with a very limited effect on RBC accessibility (d: refs 33,60), and capillary tube haematocrit increases (e: refs 19,33). These data suggest that these agonists can ‘recruit’ capillary volume for perfusion by increasing accessibility of the glycocalyx for flowing plasma, but μ-PIV measurements are needed for definite proof. Vasodilators such as adenosine, bradykinin, and SNP (f: refs 33,60) have been indicated to induce recruitment of blood-excluding glycocalyx volume in capillaries. We propose that the induced increase in capillary surface area may enable solute exchange in the capillaries to be matched to an increase in solute delivery by blood flow due to relaxation of resistance vessels during vasodilator administration and perhaps exercise as well. The insets at the bottom give a simplified illustration of the proposed constitution of the glycocalyx under control conditions and during agonist stimulation. The scaffold of the glycocalyx is formed by a mesh of anionic polysaccharide structures (proteoglycans, glycosaminoglycans, and glycoproteins) produced by the EC. In addition, association of the glycans with plasma proteins from the circulating blood permits the glycocalyx to hinder accessibility of flowing plasma and large dextrans. Agonists may increase shedding of glycosaminoglycan components (g: refs 30,82) and of plasma proteins, possibly via nitric oxide (NO) and reactive oxygen species (ROS), resulting in an increased permeation of large dextrans and presumably allowing axial plasma flow through a larger cross-section of the vessel.
Experimental research regarding the endothelial glycocalyx has erupted the last decade, and data provided have indicated that the glycocalyx constitutes a significant intravascular compartment which has an important role in endothelial homeostasis by constituting a vital barrier for cell adhesion and protein leakage and creating a depository for growth factors and enzymes. This topic has been discussed comprehensively in recent reviews.12–15 In contrast, the initial physiological role which was ascribed to the glycocalyx, i.e. its involvement in the regulation of capillary perfusion, has received less attention in recent reviews. The idea of the glycocalyx contributing to the low capillary tube haematocrit originated from the pivotal intravital microscopy studies of hamster cremaster microcirculation by Duling and co-workers.16–19 By observing that the normally low instantaneous volume fraction of red blood cells (RBCs) within capillaries, agreeing with a baseline capillary tube haematocrit of about 10%, more than doubled after local microperfusion with the enzyme heparinase, these investigators proposed the presence of a ∼1 µm-thick heparan sulfate-rich glycocalyx layer limiting functionally perfused capillary volume under resting conditions (Figure 1).17,19 These data allowed reconciliation of previous data from this group which showed that in contrast to the low haematocrits in capillary networks upon direct microscopic observation (tube haematocrit), haematocrits of the blood leaving these capillary networks (discharge haematocrit) were close to systemic haematocrit values.16,18 The classic Fåhraeus effect could well explain that the haematocrit of blood flowing through the capillaries would be decreased because of the higher concentration of RBCs near the centre of a microvessel20–22—and consequential a higher mean RBC velocity compared with plasma velocity—but it was recognized that this phenomenon could account for at most a 50% reduction in capillary tube haematocrit compared with systemic haematocrit.16,20 The data from Duling's laboratory provided evidence that exclusion of circulating RBCs and plasma by the glycocalyx was the underlying cause of the at that time common observation that capillary tube haematocrit was lower than half that of the systemic value. Additional to a role for the glycocalyx, microvascular network events, such as phase separation of RBCs and plasma at upstream bifurcations (‘network Fåhraeus effect’), have been indicated to contribute to a lowering of capillary tube haematocrit beyond 50% of the systemic haematocrit.20,22
2. The endothelial glycocalyx excludes flowing RBCs and plasma macromolecules
A more comprehensive understanding of the exclusion properties of the glycocalyx originated when the intraluminal distribution of RBCs and a variety of fluorescently labelled tracer molecules was compared with the position of endothelial wall in cremaster capillaries.23,24 In these vessels, it was observed that RBCs were normally excluded from the endothelium by a cell-free layer with a dimension of 0.4–0.6 µm but that light–dye treatment caused the RBC exclusion to be decreased to ∼0.2 µm. Light–dye treatment was associated with an increase in functionally perfused volume for blood as indicated by the 60% increase in capillary tube haematocrit (Figure 1).23 Because radical scavenging agents prohibited the light–dye effect, it was suggested that the decreased RBC exclusion was a result of a degradation of glycocalyx structures by oxygen-derived free radicals.23 A negative effect of these radicals on the exclusion properties of the glycocalyx was also suggested by the observation that administration of a bolus of oxidized lipoproteins gave similar effects on RBC exclusion and capillary tube haematocrit, while these effects were prevented by free radical scavengers as well.25,26 The reader should keep in mind, however, that light–dye and oxidized lipoproteins treatments are not truly selective stimuli for targeting the glycocalyx and were shown to include other harmful effects towards the integrity of the endothelium as well.27,28 The use of glycosaminoglycan-degrading enzymes (e.g. heparinase and hyaluronidase) seems more favourable in this regard, although non-glycocalyx-related effects upon treatment with these enzymes have also been found. Thus, exogenous hyaluronidase was shown to acutely induce the release of nitric oxide from coronary artery endothelium;29 this rapid effect, however, seems distinct from the much slower effect of hyaluronidase on hyaluronan degradation within the glycocalyx (∼30–60 min).10,30–32
Intravital microscopic evaluation of the distribution of fluorescently labelled tracers revealed that the permeation of inert macromolecules into the glycocalyx was determined by both charge and size of the macromolecules, such that in the presence of an intact glycocalyx, large (≥70 kDa) fluorescein isothiocyanate (FITC)-labelled dextrans were sterically excluded from a considerable part of the glycocalyx under control conditions, whereas smaller, neutral dextrans had seemingly unimpaired access to the entire vascular volume, including the glycocalyx (Figure 1).24,33 Light–dye treatment24 and treatment with hyaluronidase10 were shown to impair the exclusion properties of the glycocalyx, as evidenced by an increased permeation of large dextrans. Permeation of proteins into the glycocalyx did, however, not follow a simple charge- and size-dependency. Albumin (70 kDa) and fibrinogen (340 kDa) were observed to move into the glycocalyx at a similar slow rate, indicating specific interactions between these proteins and glycocalyx structures.24
3. The endothelial glycocalyx hinders transvascular solute and fluid transport
The exclusion properties permit the endothelial glycocalyx to constitute the first molecular sieve at the vascular wall. Curry and Michel34 formulated in 1980 the fibre matrix model of capillary permeability in which the size, orientation, and spacing of the fibres that form the glycocalyx were proposed to contribute to the resistance to water flow and molecular selectivity of the vessel wall. More recently, this perception has been extended into the glycocalyx-junction-break model of capillary permeability in which the selectivity of the capillary wall (pore size) is determined by the spacing between the fibres in the endothelial glycocalyx and the area for exchange (pore number) is determined by the length and frequency of breaks in the tight junction strands.35 Accordingly, the revised asymmetric Starling principle has been put forward in which the colloid osmotic forces opposing filtration across the capillary are developed across luminal and abluminal sites of the glycocalyx rather than across luminal and interstitial sites of the capillary wall.36–38 Experimental studies using enzymes to degrade glycocalyx structures have confirmed the significant contribution of an intact endothelial glycocalyx to the hindrance of fluid and solutes across the vascular wall in various organs. Thus, enzymatic treatment caused an increase in hydraulic conductivity in capillaries of frog mesentery,39–41 an increase in solute permeability of albumin and α-lactalbumin in isolated porcine coronary arterioles,42 an increase in coronary fluid filtration and the development of myocardial oedema in isolated guinea pig and rat hearts,43,44 an increased glomerular clearance of albumin in cooled isolated mouse kidneys,45 and an impaired retention of large dextrans in the systemic vasculature of hamster and mouse.31,32
4. The glycocalyx limits functionally perfused microvascular volume by impeding plasma flow: importance of both glycosaminoglycans and plasma proteins
The initial data of Duling and co-workers,17,23 showing that glycocalyx degradation was associated with an increase in capillary tube haematocrit, suggested that the presence of the glycocalyx limited functionally perfused capillary volume. In line with this proposed role of the endothelial glycocalyx as a determinant of perfused microvascular volume, it was shown that heparinase infusion in the dilated rat mesentery bed coincided with a 14–21% decrease in microvascular flow resistance.46 These authors calculated from these measures that effective vessel diameter available for RBC and plasma flow had increased by at least 0.35–0.55 µm because of the glycocalyx degradation. Studies using hydrodynamic analysis of microparticle image velocimetry (μ-PIV) data8,47–49 have been instrumental for the appreciation that in vivo functionally perfused blood volume in a vessel is reduced because axial plasma flow through the glycocalyx is greatly hindered (Figure 1). By analysing the velocity profiles of fluorescently labelled small-sized microspheres with particular emphasis on their behaviour in the region near the endothelial lining, the authors reported on ‘a hydrodynamically relevant endothelial surface layer’ (i.e. flowing blood—excluding glycocalyx) with a thickness of 0.3–0.8 µm in cremaster venules and arterioles.47–50 These measurements revealed a near-complete loss of the layer when the glycocalyx was challenged with hyaluronidase or heparinase.48,49 This impressive increase in plasma accessibility into the glycocalyx after hyaluronidase contrasts to the study of Henry and Duling,10 in which administration of this enzyme was found to only increase permeation of 70 and 145 kDa dextrans into the glycocalyx but to be without an effect on the exclusion of 580 and 2000 kDa dextrans as well as that of RBCs. These data suggest that the blood exclusion properties of the glycocalyx may result from complex interactions between polysaccharide structures and bound proteins and water molecules, and that modest changes in glycocalyx constitution may be associated with a dramatic increase in plasma accessibility, yet a less impressive increase in tracer permeation into the glycocalyx. The insight that blood-borne proteins are important contributors to the exclusion properties of the glycocalyx was also provided by μ-PIV measurements on cultured ECs. In contrast to the significant in vivo hindrance of plasma flow by a ∼0.5 µm-thick glycocalyx, Potter et al.48,49 observed no hindrance at all in cylindrical collagen microchannels that were lined with human umbilical vein ECs or bovine aortic ECs, which were maintained under standard culture conditions. In line herewith, Chappell et al.,51 using electron microscopy of perfusion-fixed tissue, reported the presence of a ∼0.9 µm-thick glycocalyx in umbilical veins taken immediately after caesarean birth, but the almost absence of glycocalyx structures on cultured human umbilical vein ECs. The authors suggested that standard cell culture conditions are not conducive to providing the environment and/or cellular conditions necessary for ECs to produce and maintain a physiologically relevant glycocalyx in vitro. Since cultured ECs, particularly when cultured under shear stress conditions,13 do produce glycocalyx-characteristic polysaccharide constituents, the data exemplify the importance of the blood-borne proteins adsorbed within the glycocalyx in governing blood exclusion.
Another important aspect of glycocalyx function, i.e. mechanotransduction of shear stress, seems to require the presence of both blood-borne proteins and the structural polysaccharide structures of the glycocalyx as well. Thi et al.52 showed that the distribution of key structural proteins in response to shear stress in confluent monolayers of ECs required the presence of albumin or serum, whereas Yao et al.53 demonstrated that, after removal of glycocalyx structures by heparinase, cultured ECs did not longer align in the direction of flow and kept proliferating after 1 day of laminar flow. Intravenous heparin infusion in a mouse, presumably causing displacement of heparan sulfate bound molecules from the glycocalyx, impaired the NO-mediated vasodilation during reactive hyperaemia in second-order arterioles of the cremaster muscle, similar to the effect of an intravenous bolus injection of hyaluronidase.31 Although various enzymes targeting different glycocalyx components were shown to impair the production of NO and NO-mediated vasodilation in arteries,54–57 the group of Tarbell and co-workers58 unravelled the contribution of the different glycocalyx constituents in shear stress-mediated NO production in cultured ECs by selectively degrading glycocalyx components from the surface of bovine aortic ECs. Whereas degradation of heparan sulfates, hyaluronan, and sialic acids from the glycocalyx all impaired fluid shear-induced NO production, removal of chondroitin sulfates was without effect.58 In contrast to the aforementioned prerequisite of proteins for shear-mediated cytoskeletal organization52 and alignment of cultured ECs,53 these cells showed a regular shear-induced NO production in media that were protein-free,5,59 suggesting that fluid shear stress can be transmitted closer to the plasma membrane when blood-borne proteins are absent in the glycocalyx and that apical signalling can proceed by mechanisms that differ from those associated with the core proteins of the glycocalyx. This topic is comprehensively discussed by Tarbell and Pahakis5 in a previous review.
5. Vasoactive substances can increase perfused capillary volume by impairing glycocalyx exclusion properties
Early intravital microscopic observations from Duling and co-workers suggested that metabolic stimuli and agonists could increase functionally perfused capillary volume by impairing exclusion of flowing blood into the glycocalyx. Capillary tube haematocrit was found to be about four-fold higher during muscle contractions and adenosine superfusion compared with the resting condition (Figure 1).19 The involvement of glycocalyx modulation was inferred by the notion that heparinase administration precluded an effect of adenosine on capillary tube haematocrit, likely because resting capillary tube haematocrit was already elevated by the enzyme.17 Furthermore, the enzyme-induced increase in resting capillary tube haematocrit occurred indifferent from a change in RBC velocity.17 More direct evidence for a true impairment of the glycocalyx exclusion properties by adenosine was provided by the intravital microscopic demonstration that FITC-labelled 70 kDa dextrans started to permeate into the glycocalyx during topical administration of the vasodilator onto the cremaster muscle.60 RBC exclusion was only modestly impaired at pharmacological doses of adenosine.60
Recently, our group showed that comparable to the effect of adenosine in the Duling studies, the vasodilators bradykinin and sodium nitroprusside (SNP) increased capillary tube haematocrit about 2.5-fold in cremaster capillaries of healthy C57Bl/6 mice.33 Although these vasodilators evoked arteriolar dilations and increases in capillary RBC velocity as well, the increase in capillary tube haematocrit occurred independently from the change in RBC velocity and seemed not the result of a hemodynamic effect of the vasodilators.33 An inverse relation between capillary tube haematocrit and RBC velocity was found under control conditions,33 and opposite to the suggested shear-dependent compression of the glycocalyx in a previous study,46 RBC exclusion appeared to actually be decreased at low velocities.33 This behaviour was also predicted by the model of Secomb et al.61 on the motion of RBCs in a glycocalyx-lined capillary using lubrication theory. Moreover, superfusion of papaverine onto the cremaster muscle at a dose that gave arteriolar vasodilation comparable to that with adenosine did not increase permeation of large dextrans into the glycocalyx at all,60 reinforcing the idea that the decrease in glycocalyx exclusion properties by bradykinin, SNP, and adenosine is due to a direct effect of these vasodilators at the level of the glycocalyx and not a result of an increased microvascular blood flow.
Impairment of the exclusion properties of the glycocalyx after bradykinin and SNP was illustrated by the observation that the increase in capillary tube haematocrit was paralleled by an increased permeation of 70 kDa dextrans into the glycocalyx.33 RBC exclusion was not affected by the vasodilators, however. An increased dextran permeation in the face of an unchanged RBC exclusion zone has been noticed in previous studies and interpreted as a manifestation of a more open or porous structure of the glycocalyx,10,60,62 whereas the robust increases in capillary tube haematocrit during vasodilator administration suggest a prominent increase in accessibility of plasma flow into the glycocalyx. To prove this, studies using hydrodynamic analysis of μ-PIV will be very helpful.
Altogether, as depicted in Figure 1, these data indicate that endothelial stimulation by vasodilator substances causes exclusion properties of the glycocalyx to be altered to allow the capillary vessel to accommodate a larger circulating blood volume. The observed two- to three-fold increases in capillary tube haematocrit during vasodilator administration require a reduction in blood-excluding glycocalyx thickness of ∼1 µm from baseline to vasodilator conditions.17,19,33 This value is comparable with the estimations by Pries et al.6,46,63 for glycocalyx thickness to explain the discrepancy between experimental estimates of apparent viscosity or flow resistance in microvessels in vitro vs. in vivo. Nevertheless, it is larger than the reported intravital microscopic glycocalyx exclusion zone for fluorescently labelled large dextrans and the hydrodynamically relevant layer from μ-PIV measures, which range from ∼0.2 to 0.8 µm,10,23,24,33,47–49,60 calling for a prudent interpretation of the vasodilator-associated increases in capillary tube haematocrit on the basis of a change in glycocalyx exclusion alone.
6. Recruitment of glycocalyx volume contributes to coronary effects of adenosine
In view of the fact that adenosine is the preferred vasodilator in the clinic for evaluation of myocardial perfusion deficits, we were interested whether an adenosine-induced impairment of the exclusion properties of the glycocalyx would be involved in coronary effects of adenosine. In anaesthetized dogs, increases in coronary conductance during adenosine hyperaemia, induced by bolus injection of adenosine, and reactive hyperaemia after 15 s occlusion of coronary arterial inflow were compared before and after glycocalyx degradation by intracoronary hyaluronidase infusion.64 It was found that under control conditions, intracoronary adenosine administration increased conductance up to 40% more than reactive hyperaemia, but that this difference was virtually abolished after hyaluronidase administration, which resulted in an increase in reactive hyperaemia without changing adenosine hyperemia.64 The observation that adenosine-induced coronary hyperaemia was insensitive to glycocalyx degradation suggested that blood exclusion by the glycocalyx was more or less negligible in the presence of adenosine and supported the hypothesis that the adenosine-induced blood volume increase in the coronary circulation includes recruitment of blood-excluding glycocalyx volume, in addition to vasodilation of resistance vessels.
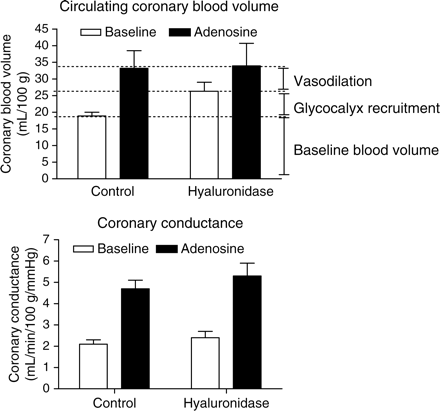
This hypothesis was recently tested in anaesthetized goats by measuring the effect of hyaluronidase treatment on the increase in circulating coronary blood volume during steady-state intracoronary adenosine infusion, measured using the tracer-dilution technique with fluorescently labelled 2000 kDa dextrans as a circulating plasma tracer and labelled RBCs as an RBC tracer.30 The results are shown in Figure 2. Maximal adenosine-mediated coronary blood volumes in the goat hearts were identical with and without prior glycocalyx degradation, indicating that the extent of adenosine's ability to impair blood exclusion by the glycocalyx was similar to that of hyaluronidase (Figure 2, top). The findings of this study, therefore, seem to confirm that recruitment of blood-excluded glycocalyx volume by an attenuation of the exclusion properties of the glycocalyx contributes significantly to the potential of adenosine to increase circulating coronary blood volume. Shedding of hyaluronan from the glycocalyx appeared to contribute to this effect since intracoronary administration of adenosine for 10–15 min resulted in a two-fold increase in the concentration of plasma hyaluronan in the coronary effluent.30
Data (mean ± SEM) adapted from Brands et al.30 in anaesthetized goats, illustrating the contribution of glycocalyx recruitment to the increase in circulating coronary blood volume (top panel) and coronary conductance (bottom panel) during intracoronary adenosine infusion. Circulating coronary blood volume was measured using the tracer-dilution technique with fluorescently labelled 2000 kDa dextrans as a circulating plasma tracer and labelled RBCs as an RBC tracer.30 Coronary conductance was defined as the ratio of coronary arterial inflow and perfusion pressure. For more methodological details, the reader is referred to the original study by Brands et al.30 Under control conditions, circulating coronary blood volume nearly doubled during adenosine infusion. After intracoronary hyaluronidase infusion, blood volume increased during baseline and was not changed during adenosine, resulting in a reduction in the adenosine-induced increase in circulating blood volume. Glycocalyx degradation did not affect coronary conductance at baseline nor during adenosine. Since it appeared that coronary conductance was increased primarily by resistance vessel relaxation (no effect of glycocalyx degradation) but that the circulating coronary blood volume increase depended on both dilation of the resistance vessels and recruitment of glycocalyx volume (increase in baseline volume without a change in adenosine-stimulated volume), the disparity between the coronary blood volume vs. flow response allows an estimation of the contribution of capillary glycocalyx volume recruitment to the total increase in circulating coronary blood volume during adenosine to be made. This is shown in the top panel: both recruitment of glycocalyx volume and vasodilation of resistance vessels appear to contribute for about 50% to the increase in circulating coronary blood volume by adenosine.
In accordance to intravital microscopic studies showing that vasodilation to adenosine in the heart occurs principally in coronary microvessels with a diameter <100 µm,65,66 we hypothesize adenosine-mediated recruitment of coronary glycocalyx volume to mainly encompass the capillary level since capillary endothelial surface area accounts for most of the total surface area of the vascular system. Consistent with this, recruitment of glycocalyx volume appeared to occur primarily in a vascular compartment with low resistance since, as in the dog experiments,64 adenosine's potential to increase coronary conductance in the goat hearts was not affected by hyaluronidase (Figure 2, bottom).30 The idea that adenosine-induced coronary hyperaemia includes augmentation of circulating blood volume in the capillary compartment has been substantiated by previous studies. Using haemoglobin-bound oxygen as an endogenous tracer, van der Ploeg et al.67 estimated total capillary volume to increase by ∼50% during maximal vasodilation with adenosine in goat hearts, whereas Liu et al.68 suggested a substantial increase in recruitable intramyocardial blood volume with an increase in blood flow during adenosine in pig hearts.
7. Glycocalyx modulation enables coupling of perfused capillary blood volume to vasodilator-induced increases in blood flow
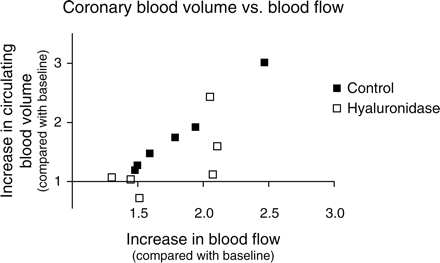
Figure 3 shows the role of a functional glycocalyx in coupling circulating coronary blood volume to adenosine-induced increases in coronary blood flow. With an intact glycocalyx (closed symbols), the increase in coronary blood flow appears to be nicely matched by an increase in circulating blood volume; this relation is lost after hyaluronidase treatment (open symbols). Agonist-induced recruitment of blood-excluding glycocalyx volume in capillaries in parallel to relaxation of resistance vessels may enable coordination of substrate exchange in the capillaries to substrate delivery by blood flow. It has been recognized in many studies that increases in flow in cardiac and skeletal muscle are commonly accompanied by increases in the capillary permeability–surface area product (PS product), and this increase in PS product has mainly been explained on the basis of an increase in surface area by an increased number of perfused capillaries (‘capillary recruitment’).69–71 Recruitment of glycocalyx volume may increase functionally perfused volume in an individual capillary that is already being perfused, thereby providing an alternative mechanism for the phenomenon of capillary recruitment (Figure 1).72 Besides the increase in capillary surface area, a concerted effect of vasodilator substances on blood flow and microvessel permeability per se has been noticed in the past as well.73 Given the strategic position of the endothelial glycocalyx as the initial intraluminal sieve of the vascular wall, it is not unconceivable that attenuation of the exclusion properties by vasoactive substances might also affect solute permeability, but more studies are needed in this respect. From our recent coronary blood volume measurements in the goat hearts, yet another potential important facet of the contribution of agonist-induced impairment of glycocalyx exclusion to the exchange capacity at the capillary level appeared. Under control conditions, i.e. in the presence of an intact glycocalyx, the transit time for RBCs and dextrans through the coronary system was surprisingly well maintained during adenosine, despite the robust increase in blood flow. In contrast, after hyaluronidase treatment, RBC transit time decreased during adenosine infusion compared with baseline transit time.30 These data suggest that the adenosine-induced recruitment of glycocalyx volume causes a slowing down of the RBCs in the capillaries in the face of the increase in arterial blood flow. Consistent with this finding, direct observations of epicardial coronary capillary haemodynamics in the canine heart showed that a 4.2-fold increase in blood flow with adenosine was associated with only modest increases in capillary RBC velocity in the epicardial capillaries.74
Adenosine-induced increases in circulating coronary blood volume (Y-axis) vs. coronary blood flow (X-axis) in anaesthetized goat hearts during control conditions (closed symbols) and after intracoronary administration of hyaluronidase (open symbols). Each data point represents an individual experiment in the study of Brands et al.,30 of which average data are shown in Figure 2. Blood volumes and flows are normalized to their corresponding baseline values. In control hearts, the increase in blood flow corresponds with an increase in blood volume (R2 = 0.99). After hyaluronidase treatment of the glycocalyx, this match between flow and volume seems virtually absent.
8. Clinical implications and opportunities
Angina during exercise has been generally related to a jeopardized macrocirculation, in particular to the presence of a flow-limiting stenosis in one of the large coronary arteries. Measurement of coronary flow reserve using adenosine as vasodilator is common clinical practice to determine the functional relevance of such a stenosis. A considerable number of patients with symptoms of chest pain during exercise, however, do not show a prominent stenosis in their coronary arteries or no coronary artery disease at all.75 Furthermore, a large number of the patients with a detectable lesion have a haemodynamically non-significant intermediate coronary artery stenosis, and these patients do not benefit from a percutaneous coronary intervention.76 In all these cases, the angina may relate to coronary microvascular dysfunction,77 of which damage to the endothelial glycocalyx may be an early trigger.14,78 Our experimental findings suggest that measurement of coronary blood volume reserve using adenosine as ‘glycocalyx recruiting agonist’ may identify a damaged glycocalyx, whereas measurement of coronary flow reserve appears to be insensitive to glycocalyx injury (Figure 2).30,64 Thus, non-invasive methods that measure coronary microvascular volume, such as magnetic resonance imaging or contrast-enhanced ultrasound using microbubbles may be promising to detect early coronary microvascular vulnerability in patients with angina but without overt coronary artery disease. These patients might then benefit from therapeutic interventions aiming at a restoration of the balance between glycocalyx degradation and production by limiting exposure of the glycocalyx to vulnerable conditions on the one hand and supplementation of a damaged glycocalyx on the other hand.79
9. Physiological role for agonist-induced modulation of glycocalyx exclusion?—remaining issues and final remarks
Exercise constitutes a challenge that requires optimization of blood flow and solute flux to match exchange with tissue demand. In this respect, functional recruitment of glycocalyx volume upon an increase in metabolic demand of muscle was inferred by Klitzman and Duling19 when they noted that capillary tube haematocrit doubled during contractions of the cremaster muscle. Besides the fact that the role of endogenously produced vasoactive substances for exercise hyperaemia in heart and skeletal muscle is uncertain,80,81 a distinct lack of information on both the cellular mechanisms and temporal dynamics of glycocalyx alterations in response to agonist stimulation preclude current appreciation of a potential physiological role of glycocalyx modulation in matching exchange capacity to changes in metabolic demand. Although rapid changes in glycocalyx exclusion may be suggested by the commonly observed moment-to-moment variations in capillary tube haematocrit in cremaster muscle,16 such a rapid time course is difficult to reconcile with the available data on the dynamics of glycocalyx modulation and recovery, which suggest the time course to be much slower. Studies determining effects of vasodilators on glycocalyx exclusion of large dextrans have so far considered only steady-state conditions and found increases in permeation to occur within 10–15 min,33,60 whereas maximal impairments in glycocalyx exclusion after a bolus of oxidized LDL25 and enzymatic treatment10,32 were observed after ∼30–60 min. Recovery of the glycocalyx exclusion properties took about 30 min in those studies. In the goat heart, recovery of the glycocalyx after adenosine was inferred from the finding that baseline coronary blood volume was unaltered after adenosine had been washed out; baseline coronary blood volume was measured not earlier than 1 h after the adenosine infusion had ended, however.30
More studies are also needed to delineate the cellular mechanisms by which vasoactive substances may modulate exclusion properties of the glycocalyx. The actual state of the glycocalyx is dependent on the intrinsic properties and interactions of its constituents, the polysaccharides, and blood-borne proteins, as well as the local microenvironment, such as cation content and pH.5,8 Shedding of glycosaminoglycan fragments from the coronary glycocalyx was suggested during stimulation with adenosine30 and stimulation with atrial natriuretic peptide.82 Platts and Duling60 showed strong evidence that adenosine A3 receptor activation was involved in the effect of adenosine in the cremaster muscle and suggested a role for mast cell activation and reactive oxygen species degrading hyaluronan from the glycocalyx. On the other hand, the effects of bradykinin and SNP in the cremaster experiments33 may suggest a role for NO and its second messenger cGMP.83 Besides degradation of heparan sulfates in response to NO, we have suggested an alternative explanation for the effect of NO on the glycocalyx, namely a change in its charge density.33 Finally, in light of the important role of the glycocalyx in the mechanotransduction of shear stress to the EC, it would be interesting to see whether agonist-induced modulation of glycocalyx exclusion properties would also affect the process of shear-dependent NO production.
The current review has evaluated the glycocalyx as circulating blood-excluding intravascular compartment, which appears to be sensitive to modulation by vasoactive agents. On the basis of recent experimental data, the concept has been put forward that vasodilator substances may increase circulating blood volume in capillaries by impairing blood exclusion by the glycocalyx as means of coordinating substrate exchange to substrate delivery by blood flow. On the other hand, impairment of the glycocalyx exclusion properties by enzymatic treatment has been shown to confront the vessel with a diminished protection to adhering platelets and leucocytes, transvascular protein and fluid leakage and associated oedema formation, and a reduction in shear-mediated NO bioavailability.5,12,14,78 Glycocalyx damage has been inferred to be associated with an impaired capacity for agonist-induced recruitment of glycocalyx volume for plasma perfusion, simply because of the impaired exclusion under resting conditions already.17,78 Compared with control mice, baseline capillary tube haematocrit in hyperlipidaemic mice was found to be elevated, and subsequent bradykinin and SNP administration did not further increase it.33 In a similar manner, hyaluronidase-treated goat hearts had increased resting coronary blood volumes, causing the remaining volume increase during adenosine administration to be reduced (Figure 2). These data underline the importance of a functional glycocalyx in the microcirculation under resting conditions because it determines the extent to which capillary volume and exchange capacity can be adapted to match increases in blood flow in the body. It seems essential, however, that the capacity for recruitment of glycocalyx volume is controlled to some extent, since it seems to appear at the expense of a diminished protection of the endothelium.
Conflict of interest: none declared.
Funding
Our work is supported by the Netherlands Heart Foundation (grant numbers 2003B181, 2005T037, 2009B056) and the Dutch Diabetes Research Foundation (grant number 2006.00.027).
References
Author notes
This article is part of the Spotlight Issue on: ‘Microvascular Permeability’