-
PDF
- Split View
-
Views
-
Cite
Cite
Sowndramalingam Sankaralingam, Han Xu, Sandra T. Davidge, Arginase contributes to endothelial cell oxidative stress in response to plasma from women with preeclampsia, Cardiovascular Research, Volume 85, Issue 1, 1 January 2010, Pages 194–203, https://doi.org/10.1093/cvr/cvp277
Close - Share Icon Share
Abstract
Preeclampsia is a hypertensive disorder characterized by vascular oxidative stress. Decreased availability of the vasodilator nitric oxide (NO) has been postulated to be involved in the pathophysiology of this disorder. Arginase, an enzyme that competes with nitric oxide synthase (NOS) for l-arginine, not only reduces NO formation but also increases superoxide production by NOS. In placenta of preeclamptic women, arginase upregulation has been shown to be increased and contributes to superoxide formation via uncoupling of NOS. However, the role of arginase in the maternal vasculature is not clear. We hypothesized that arginase would be upregulated in the maternal vasculature of women with preeclampsia and contribute to oxidative stress within the endothelium.
We observed increased arginase expression in the maternal vasculature of women with preeclampsia compared with normotensive pregnant women. Furthermore, human umbilical vein endothelial cells treated with 2% plasma from preeclamptic women show increased arginase II expression and activity that was reduced by a peroxynitrite scavenger. Also, both 3-morpholino sydnonimine and exogenous peroxynitrite increased arginase expression and activity. Preeclamptic plasma treatment increased superoxide and peroxynitrite levels. Superoxide levels were significantly reduced after arginase and NOS inhibition with [(S)-(2-boronoethyl)-l-cysteine] and Nω-nitro-l-arginine methyl ester, respectively, but peroxynitrite levels were in fact increased after arginase inhibition. Moreover, in the presence of preeclamptic plasma, l-arginine supplementation increased peroxynitrite formation during arginase inhibition.
Increased arginase expression in preeclampsia can induce uncoupling of NOS as a source of superoxide in the maternal vasculature in preeclampsia. However, l-arginine supplementation in the face of oxidative stress could lead to a further increase in peroxynitrite.
1. Introduction
Pregnancy is a state of enhanced vasodilation partly mediated by the endothelium-derived vasodilator, nitric oxide (NO),1,2 which serves to reduce vascular resistance in order to accommodate the increased plasma volume that occurs during pregnancy. This cardiovascular adaptation to pregnancy may be impaired in women with preeclampsia leading to hypertension and proteinuria that is characteristic of this syndrome.3 One manifestation of preeclampsia is oxidative stress,4,5 which could impact on the NO system.
Nitric oxide is synthesized from the amino acid l-arginine by the enzyme NO synthase (NOS).6 It has been hypothesized that a reduced bioavailability of NO, either because of a decreased formation of NO or increased scavenging of NO by superoxide, can result in reduced vasodilation that is observed in preeclampsia.7 We have previously shown that eNOS protein expression is increased in the vasculature of women with preeclampsia.8 However, in the face of oxidant stress during preeclampsia, the NO generated could react with superoxide and generate excess peroxynitrite. Indeed we have also previously observed increased peroxynitrite formation in the maternal vasculature of women with preeclampsia.8 Moreover, we have observed that preeclamptic plasma can increase peroxynitrite formation in endothelial cells in culture due to the effect of circulating factors. While evidence for increased superoxide has been documented in the maternal vasculature of women with preeclampsia,8 the enzymatic source of superoxide in the maternal vasculature that may be enhanced due to circulating factors is still unclear. Among the various enzymatic sources of superoxide, NADPH oxidase and NOS are major sources in the endothelium.9 In our previous study, we have shown that NADPH oxidase contributed to about half of all superoxide generated in endothelial cells in response to plasma from women with preeclampsia. NOS is also regarded as another major source of superoxide.9 NOS generates superoxide when it undergoes a phenomenon called uncoupling. All isoforms of NOS can undergo uncoupling due to deficiency of either l-arginine, the substrate or co-factors such as tetrahydrobiopterin (BH4)10 which results in decreased NO formation and increased superoxide levels.11 Reduced levels of l-arginine can occur when there is increased expression of arginase, an enzyme that competes with NOS for l-arginine,12 which can result in the formation of reactive oxygen species such as superoxide and subsequently peroxynitrite.13
Arginase exists in two isoforms, viz arginase I (cytosolic) and arginase II (mitochondrial). The primary function of arginase I and II in the liver and kidney, respectively, are to regulate urea metabolism.14 In the vasculature, arginase I is expressed both in vascular smooth muscle cells and the endothelium,15,16 while arginase II is highly expressed in the endothelium.17–19 Recent studies suggest that inhibiting arginase can improve vascular function in aging rats,15 as well as in spontaneously hypertensive rats.20 Moreover, arginase II expression has been shown to be increased in the placenta of women with preeclampsia.13 These studies postulate that arginase inhibition leads to more l-arginine availability and therefore more NO formation and availability which favours vasorelaxation and hence reduction in blood pressure. However, clinical trials using l-arginine supplementation to enhance NO formation and reduce blood pressure in preeclamptic women have been conducted with limited success.21–23 Thus the mechanisms of l-arginine depletion and the impact of supplementation is likely more complex than just replenishing NO during states of increased oxidative stress.
In this study, we hypothesized that arginase expression is increased in the vasculature of women with preeclampsia. To address the mechanisms of arginase upregulation in preeclampsia, we hypothesized that circulating factors in the plasma of women with preeclampsia would upregulate arginase in endothelial cells in culture and contribute to oxidative stress. We further evaluated the effect of arginase inhibition or l-arginine supplementation on oxidative stress markers in isolated endothelial cells.
2. Methods
2.1 Subjects
Non-pregnant, pregnant, and preeclamptic subjects (12 per group) were recruited for this study at the Royal Alexandra Hospital, Edmonton, Canada. The protocols were approved by the University of Alberta Ethics Committee (Study ID # MS1_Pro00000880) and the studies were conducted according to the principles of the Declaration of Helsinki. Omental fat biopsies were obtained from these subjects during abdominal surgeries or caesarean section and stored at −80°C. Details of the inclusion and exclusion criteria and the subject characteristics are provided in the Supplementary material online.
2.2 Immunohistochemistry
Our first aim was to assess the expressions of arginase I and II in the vasculature of non-pregnant, pregnant, and preeclamptic women. The methodological details are provided in the Supplementary material online.
2.3 Experimental protocol
To address the mechanisms of the observed arginase II upregulation, we performed a bioassay by treating endothelial cells in culture with plasma from these three groups of women. For our study, we selected human umbilical vein endothelial cells (HUVECs) because they are of human origin, easily available, and although of foetal origin, they are in a pregnant milieu. Importantly, they express arginase and its reciprocal enzyme NOS, thereby serving as an excellent tool to examine the responses to plasma. However, it is noted that HUVECs have their limitations, since they are foetal-derived cells. These endothelial cells were treated with 2% plasma from non-pregnant, pregnant, and preeclamptic women for 24 h. This dose and timepoint was chosen based on our previous studies.24–27
We first sought to compare arginase expression and activity in response to plasma. Additional experiments were designed to determine if oxidant stress could be a mechanism for arginase upregulation. We have previously observed increased nitrotyrosine staining (a marker for peroxynitrite formation) in endothelial cells in response to 2% plasma from women with preeclampsia.27 Since peroxynitrite activates NFκB translocation28 and that increased arginase expression itself activates NFκB,29 we hypothesized that peroxynitrite generated in response to preeclamptic plasma could be involved in the upregulation of arginase by providing a feedforward mechanism. Therefore, in some experiments, we have used FeTPPS (5 µmol/L),27 a peroxynitrite scavenger in the presence of preeclamptic plasma to investigate if peroxynitrite generated by preeclamptic plasma was responsible for enhanced arginase expression and activity. To further confirm the effects of peroxynitrite, SIN-1 a peroxynitrite donor (SIN-1 generates both superoxide and nitric oxide within the cells which react to form peroxynitrite) or exogenous peroxynitrite were used to treat cells. Endothelial cells were treated with SIN-1 (0.25 mmol/L) for 12 h. Also, cells were treated with exogenous peroxynitrite (25 µmol/L) for 6 h. These doses and timepoints were chosen based on our previous studies27,28 as well as our own preliminary experiments specific for this data set. Higher doses and longer incubation periods resulted in cell death. We also used inhibitors of NFκB activation, BAY 11-7082 (BAY, 5 µmol/L),30 and caffeic acid phenylethyl ester (CAPE, 25 µg/mL),31 in the presence of SIN-1 and assessed both arginase expression and activity. Since, NOS competes with arginase for l-arginine, we also compared eNOS and iNOS expression and activity. NOS expression was assessed by western blot and the activity was compared by two different approaches. The activity was compared by a radioactive assay that measures l-citrulline formation from 14C-labelled l-arginine and also by measuring the total nitrite/nitrate levels in the cell-culture media. Detailed methods are provided in the Supplementary material online.
To examine the effects of plasma on endothelial cells with respect to oxidative stress, we measured both superoxide and peroxynitrite levels. In these experiments, to examine the contribution of arginase we have used BEC (100 µmol/L),32,33 a potent and specific inhibitor of arginase.32 To investigate the role of NOS, we used l-NAME (100 µmol/L), an inhibitor of all isoforms of NOS and 1400W (100 µmol/L) a relatively specific iNOS inhibitor at the dose used.34 At times when NOS is suspected as a source of superoxide, use of l-NAME to inhibit NOS and measure superoxide levels is a commonly used method to identify uncoupling of NOS.35 We have also used BH4 (10 µmol/L)36 a co-factor for NOS, l-arginine (2 mmol/L)37 the substrate for NOS and DPI (10 µmol/L), an NADPH oxidase inhibitor to either augment or inhibit certain pathways to determine their role in superoxide and/or peroxynitrite generation. Control experiments conducted using l-arginine (2 mmol/L) alone on endothelial cells did not increase superoxide or nitrotyrosine levels. All chemical compounds were dissolved in distilled water except CAPE and BAY 11-7082 which were dissolved in DMSO and later diluted in isotonic saline solution. The final concentration of DMSO was 0.0001%. BH4 was dissolved in 0.1N HCl to prevent spontaneous oxidation.
The specific experimental details of the techniques used in this study such as, western blot, arginase activity assay are provided in the Supplementary material online.
2.4 Statistical analysis
Values are expressed as mean ± SEM. Comparison of three or more groups was done using a one-way ANOVA followed by a Tukey's post hoc test. In some experiments, due to the experimental design, Bonferroni's correction was applied. Comparison of two groups was conducted using a Student’s t-test. A P-value of <0.05 was deemed significant. A two-way ANOVA was used to compare NOS activity in the presence or absence of BEC, the arginase inhibitor.
3. Results
3.1 Arterial expression of arginase
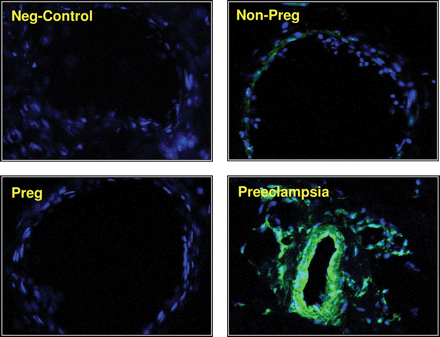
We observed abundant arginase II expression in arteries from women with preeclampsia (13.25 ± 0.6 Arbitrary Units (AU); P < 0.01), while there was minimal expression in arteries from non-pregnant women (1.13 ± 0.01 AU). Interestingly, arginase expression in pregnant women was negligible (0.007 ± 0.001 AU) (Figure 1). Arginase I expression was similar in the maternal vasculature of non-pregnant, pregnant, and preeclamptic women (data not shown).
Arginase expression in the maternal vasculature. Immunohistochemical staining for arginase II expression in representative sections of small arteries from omental fat biopsies of non-pregnant, pregnant, and preeclamptic women shown at magnification ×200. Shown also is a small artery obtained from preeclamptic woman as a negative control that received rabbit IgG.
3.2 Endothelial response to plasma
3.2.1 Arginase II expression and activity
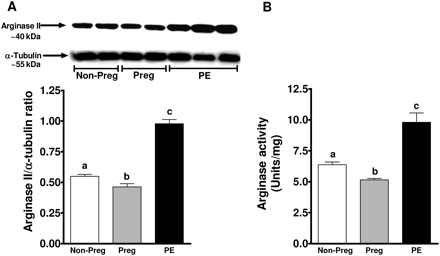
Arginase II expression was significantly increased in endothelial cells exposed to plasma from women with preeclampsia (0.98 ± 0.03 AU; P < 0.05) in comparison to cells treated with plasma from non-pregnant and pregnant women (0.54 ± 0.01 and 0.46 ± 0.02 AU), respectively (Figure 2A). Arginase activity paralleled increases in arginase expression. Plasma from women with preeclampsia significantly increased arginase activity when compared with treatment with non-pregnant and pregnant plasma (9.8 ± 0.8 vs. 6.4 ± 0.21 and 5.1 ± 0.12 Units/mg protein, P < 0.05) (Figure 2B).
Arginase expression and activity in response to plasma. (A) A representative Western blot for arginase II expression from endothelial cells treated for 24 h with 2% plasma from non pregnant, pregnant, and preeclamptic women. Summary graph shows densitometric analysis of arginase II expression normalized to α-tubulin from 6 samples in each group. (B) Summary graph shows arginase activity in response to treatment with plasma from three groups of women. Bars represent means ± SEs. Different letters denote significant difference (P < 0.05) from each other.
3.2.2 NOS expression and activity
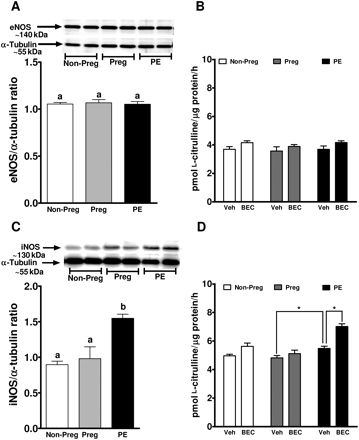
Treatment of endothelial cells with plasma from non-pregnant, pregnant, or women with preeclampsia did not affect eNOS protein expression (Figure 3A). Interestingly, iNOS expression was significantly increased by treatment with plasma from women with preeclampsia (1.54 ± 0.07 AU; P < 0.05) when compared with treatment with plasma from non-pregnant (0.99 ± 0.05 AU) and pregnant women (0.91 ± 0.16 AU; Figure 3C).
eNOS and iNOS expression and activity in response to plasma. A representative western blot showing (A) eNOS and (C) iNOS expression from endothelial cells treated for 24 h with 2% plasma from non-pregnant, pregnant, and preeclamptic women. Summary graphs show densitometric analyses of eNOS and iNOS expressions normalized to α-tubulin from six samples in each group. Bars represent means ± SEs. Different letters denote significant difference (P < 0.05) from each other. Also shown are the calcium-dependent (B) and calcium independent (D) NOS activity expressed as the formation of l-citrulline in response to plasma from three groups of women in the presence or absence of BEC, the arginase inhibitor. *P < 0.05.
Calcium-dependent NOS activity was similar in cells treated with non-pregnant, pregnant, and women with preeclampsia (Figure 3B). Arginase inhibition resulted in a small (∼15–20%) increase in NOS activity in non-pregnant and preeclamptic groups, but very minimal increase in cells exposed to pregnant plasma. Calcium-independent NOS activity (iNOS) was significantly increased in cells treated with plasma from women with preeclampsia when compared with pregnant plasma treatment (4.81 ± 0.09 vs. 5.49 ± 0.07 pmol l-citrulline/µg/h, P < 0.05; Figure 3D). BEC, the arginase inhibitor significantly increased iNOS activity only in the cells treated with plasma from women with preeclampsia (Figure 3D).
Total nitrite/nitrate levels measured in the cell-culture supernatant were similar after treatment with non-pregnant, pregnant, and preeclamptic women. However, the levels were significantly increased after arginase inhibition only in the preeclamptic group compared with the pregnant group (0.066 ± 0.002 vs. 0.081 ± 0.003 µmol/L, P < 0.05).
3.2.3 Effect of peroxynitrite on arginase expression
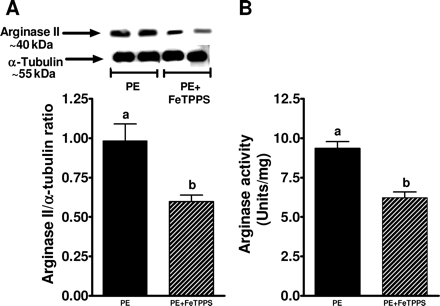
In a separate set of experiments, cells treated with preeclamptic plasma were also incubated with FeTPPS, a specific peroxynitrite scavenger (Figure 4). FeTPPS significantly reduced PE plasma-induced arginase II expression and activity by ∼40% (P < 0.05).
Arginase expression and activity in response to preeclamptic plasma and FeTPPS. (A) A representative western blot for arginase II expression from endothelial cells treated for 24 h with 2% plasma from preeclamptic women in the presence or absence of FeTPPS, a peroxynitrite scavenger. Summary graph shows densitometric analysis of arginase II expression normalized to α-tubulin from six samples in each group. (B) Graph shows arginase activity in response to preeclamptic plasma in the presence or absence of FeTPPS. Bars represent means ± SEs. Different letters denote significant difference (P < 0.05) from each other.
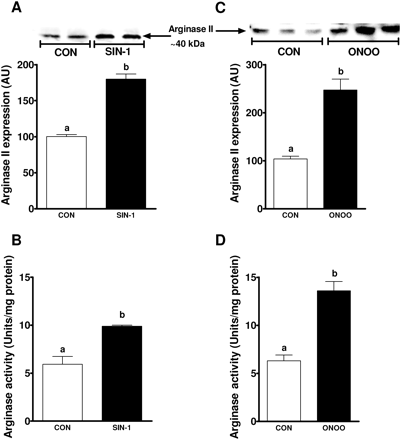
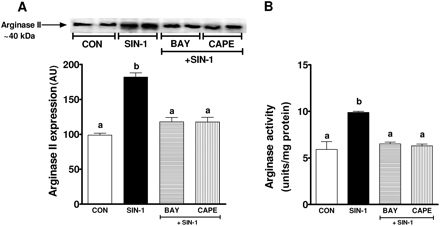
To further identify the role of peroxynitrite on arginase expression, endothelial cells treated with SIN-1 significantly increased both arginase II expression (100 ± 1 vs. 180 ± 5 AU; P < 0.01) and activity (5.9 ± 0.8 vs. 9.89 ± 0.2 Units/mg protein; P < 0.05) compared with no treatment (Figure 5A and B). These effects were confirmed with exogenous peroxynitrite which induced a robust increase in arginase II expression (103.5 ± 1.5 vs. 247.7 ± 23 AU; P < 0.01) and activity (6.3 ± 0.6 vs. 13.6 ± 0.9 Units/mg protein; P < 0.05) (Figure 5C and D). To further examine the mechanisms of peroxynitrite-induced arginase II expression, NFκB was inhibited using two different inhibitors, BAY and CAPE. Both BAY and CAPE significantly reduced SIN-1-induced arginase II expression and activity (Figure 6A and B). Inhibitors of NFκB alone did not affect arginase II expression (data not shown).
Effect of SIN-1 and exogenous peroxynitrite on arginase expression and activity. (A and C) Shown are representative western blots of arginase II expression in response to (A) SIN-1 and (C) ONOO- treatment on endothelial cells for 12 and 6 h, respectively. Summary graphs show densitometric analyses of arginase II expression from three independent experiments. (B and D) Graphs show arginase activity in response to (B) SIN-1 and (D) ONOO- treatment from three independent experiments. Bars represent means ± SEs. Different letters denote significant difference (P < 0.05) from each other.
Role of NFκB in SIN-1-induced arginase expression and activity. (A) Shown is a representative western blot for arginase II expression in response to SIN-1 in the presence of BAY 11-7082 and CAPE, NFkB inhibitors. Summary graph shows densitometric analysis of arginase II expression from three independent experiments. (B) Summary graph showing inhibition of arginase activity by NFκB inhibitors BAY 11-7082 and CAPE in response to SIN-1. Bars represent means ± SEs. Different letters denote significant difference (P < 0.05) from each other.
3.2.4 Effect of plasma and arginase inhibition on markers of oxidative stress
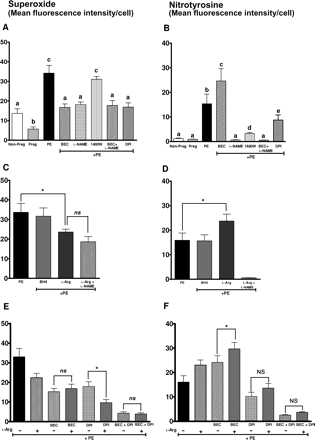
Our aim was to investigate if arginase and therefore uncoupling of NOS contributes to oxidative stress in response to plasma from women with preeclampsia. Therefore, in our first set of experiments (Figure 7A and B), we observe that treatment of endothelial cells with plasma from women with preeclampsia significantly increased superoxide generation (34.2 ± 3.9, P < 0.05) when compared with treatment with plasma from pregnant (5.7 ± 1.1) and non-pregnant women (13.66 ± 2.4) (Figure 7A). Plasma from women with preeclampsia also increased nitrotyrosine staining in parallel with increases in superoxide levels (Figure 7B). Also, inhibition of arginase or eNOS with BEC or l-NAME, respectively, significantly reduced superoxide generation (Figure 7A). Interestingly, inhibition of arginase increased nitrotyrosine staining (Figure 7B). The iNOS inhibitor 1400W did not affect superoxide levels, but significantly reduced nitrotyrosine staining (Figure 7A and B). Also, l-NAME completely abolished nitrotyrosine staining (Figure 7B). Importantly l-NAME (inhibitor of all isoforms of NOS) reduced superoxide levels significantly more than that by 1400W (iNOS). Also, DPI, an NADPH oxidase inhibitor reduced superoxide levels and nitrotyrosine staining (Figure 7A and B) further confirming NADPH oxidase as another source of superoxide as shown in our previous work.27 Thus our initial set of experiments confirms that arginase and therefore uncoupling of eNOS is a source of superoxide in endothelial cells in response to preeclamptic plasma.
Effect of plasma on oxidative stress markers. (A and B) Graph shows (A) superoxide levels and (B) nitrotyrosine staining in response to plasma from three groups of women and in the presence of arginase inhibitor BEC, NOS inhibitor l-NAME, iNOS inhibitor 1400W, and NADPH oxidase inhibitor DPI. (C and D) Graphs show (C) superoxide levels and (D) nitrotyrosine staining in response to plasma from women with preeclampsia (PE) with BH4 and l-arginine supplementation. (E and F) Graphs show (E) superoxide levels and (F) nitrotyrosine staining in endothelial cells treated with preeclamptic plasma in the presence of arginase inhibitor BEC or NADPH oxidase inhibitor DPI or both with or without l-arginine supplementation. Bars represent means ± SEs. Different letters denote significant difference (P < 0.05) from each other. In panels (C) and (D), statistical analysis was conducted using Bonferroni's correction. Asterisk indicates P < 0.05. In panels (E) and (F), the bar representing preeclampsia (PE) is used only for reference and was not included in the statistical analysis. A one-way ANOVA was performed to compare differences between the other six groups. Asterisk represents P < 0.05, while n.s. represents not significant.
In the second set of experiments (Figure 7C and D), we sought to determine the cause of uncoupling of NOS. Uncoupling of NOS can occur due to decreased co-factor levels (BH4) or reduced substrate, l-arginine levels. We have therefore supplemented either BH4 or l-arginine in the presence of preeclamptic plasma and determined superoxide and nitrotyrosine staining. In the presence of preeclamptic plasma, supplementation of excess BH4 did not affect either superoxide or nitrotyrosine staining (Figure 7C and D). Interestingly, in response to preeclamptic plasma, l-arginine supplementation significantly reduced superoxide levels (Figure 7C) but increased nitrotyrosine staining (Figure 7D). However, l-arginine+l-NAME did not further reduce superoxide levels above and beyond the effects of l-arginine alone (Figure 7C). l-Arginine + l-NAME completely abolished nitrotyrosine staining (Figure 7D).
In the third set of experiments (Figure 7E and F), we wanted to examine the effect of l-arginine supplementation on superoxide levels and nitrotyrosine staining in the presence of preeclamptic plasma with and without inhibition of sources of superoxide. Although arginase inhibition was not further altered by l-arginine (Figure 7E), DPI (NADPH oxidase inhibitor) reduced superoxide levels that was further reduced by l-arginine supplementation (Figure 7E). Thus DPI directly inhibits superoxide generation via NADPH oxidase, supplementation with l-arginine further rescues uncoupling of NOS resulting in greater reduction in superoxide levels. However, l-arginine supplementation did not affect superoxide levels after inhibition of both arginase and NADPH oxidase. Similar to experiments measuring superoxide, we further confirmed the effects of arginase or NADPH oxidase inhibition or both on nitrotyrosine staining in Figure 7F. Although l-arginine did not affect superoxide levels after arginase inhibition with BEC (Figure 7E) l-arginine supplementation, in fact increased nitrotyrosine staining in the presence of arginase inhibitor. However, l-arginine supplementation did not affect nitrotyrosine levels in the presence of NADPH oxidase inhibitor or arginase+NADPH oxidase inhibition (Figure 7F). This set of experiments provides evidence for increased peroxynitrite formation during l-arginine supplementation in the face of oxidative stress. In this case, even after arginase inhibition with BEC, superoxide generation continues unabated mainly via NADPH oxidase and therefore excess l-arginine can lead to increased NO production and ultimately results in the observed increased formation of nitrotyrosine.
4. Discussion
In this study, we demonstrated an increased arginase II expression in the vasculature of women with preeclampsia. In addition, endothelial cells incubated with plasma from women with preeclampsia show enhanced arginase II expression and activity as well as increased superoxide levels and evidence for increased peroxynitrite formation (nitrotyrosine staining). We also showed that peroxynitrite via NFκB could increase arginase expression and activity. Interestingly, although inhibition of arginase reduced superoxide levels, it led to increased nitrotyrosine staining. In the presence of preeclamptic plasma, addition of excess l-arginine also leads to increased nitrotyrosine staining, unless co-incubated with an NADPH oxidase inhibitor to reduce an alternate source of superoxide. Thus, l-arginine supplementation in the face of oxidative stress can result in deleterious effects due to its possible role to enhance formation of peroxynitrite.
Arginase is an enzyme of the hepatic urea cycle involved in the detoxification of urea and functions in the formation of polyamines such as spermine, spermidine and putrescine that are involved in cell growth, proliferation, and wound healing.14 In fact, increased arginase expression has been noted in normal pregnancies at the implantation site in guinea pigs, rats, and sheep which may serve to provide precursors for protein synthesis that is much needed for a growing foetus.38–40 Thus, arginase does have a physiological role in pregnancy. However, increased arginase expression in the vasculature of women with preeclampsia can compete with NOS for l-arginine which can have important implications for vascular dysfunction.41 Therefore, an upregulation of arginase can result in excess consumption of l-arginine, the substrate for NOS, resulting in decreased l-arginine that is available for NOS. Ultimately this could lead to uncoupling of NOS that generates superoxide instead of NO and therefore can act as a source of oxidant stress.
We also show in this study that plasma from women with preeclampsia increase arginase expression and activity when compared with plasma from normotensive pregnant women. FeTPPS, a peroxynitrite scavenger, significantly reduced the arginase expression that was induced by preeclamptic plasma suggesting that peroxynitrite generated by preeclamptic plasma is responsible for the upregulation of arginase. Indeed, we have previously demonstrated increased peroxynitrite formation in endothelial cells incubated with preeclamptic plasma27 and also increased evidence of peroxynitrite in the vasculature of preeclamptic women.8 We further identified that, SIN-1 or exogenous peroxynitrite can upregulate arginase expression and activity via NFκB. Thus arginase expression results in enhanced peroxynitrite formation, which provides a feedforward loop to further upregulate arginase. This feedforward progression may lead to the worsening of a condition which is typical of the symptoms for preeclampsia where delivery is currently the only treatment option.
As previously noted, arginase is known to compete with NOS for l-arginine. Arginase and NOS share the same intracellular pool of l-arginine and therefore, an increased expression of arginase could affect l-arginine bioavailability for NOS.42 Thus decreased availability of the substrate, l-arginine could lead to uncoupling of NOS, resulting in the generation of superoxide by NOS.43 This is supported by our experiments in which inhibition of NOS by l-NAME reduces superoxide levels, thus confirming NOS as a source of superoxide in response to plasma from women with preeclampsia. We also find that calcium-dependent NOS activity (eNOS and nNOS) did not differ between groups; however, arginase inhibition increased NOS activity in the non-pregnant and preeclamptic groups but not in the pregnant group. This further confirms our eNOS protein expression data showing no differences in eNOS protein expression. Since, arginase and NOS compete for l-arginine as a substrate, inhibition of arginase with BEC resulted in increased NOS activity. On the other hand, calcium-independent NOS activity (iNOS) was increased after treatment with preeclamptic plasma when compared with non-pregnant and pregnant plasma, mirroring iNOS protein expression. Also, NOS activity was significantly increased after arginase inhibition, only in the preeclamptic group, further confirming a significantly increased arginase expression in cells treated with plasma from preeclamptic women only. Thus, an already increased iNOS expression in endothelial cells exposed to preeclamptic plasma, leads to increased formation of l-citrulline when provided l-arginine after arginase inhibition. Uncoupling of NOS can occur as a result of cofactor or substrate deficiency.11,43 We therefore used excess BH4 or l-arginine in the cell-culture media. We did not observe significant changes in preeclamptic plasma-induced superoxide or nitrotyrosine staining after BH4 supplementation, suggesting that the uncoupling of NOS was not likely due to BH4 depletion. The cause of uncoupling of NOS appears to be an upregulation of arginase, in that arginase inhibition which makes l-arginine available for NOS, reduces superoxide levels. Inhibition of arginase or NOS reduced superoxide by similar levels, moreover inhibition of both arginase and NOS did not have an additive effect, suggesting that both are acting through a common pathway and that arginase is responsible for uncoupling of NOS. Diminished uptake of l-arginine can also result in uncoupling of NOS.44 However, the total nitrite/nitrate levels, a marker for NOS activity in the cell-culture supernatant did not differ after treating live intact endothelial cells with plasma from three groups of women, suggesting that diminished l-arginine uptake in response to plasma was not a likely cause for uncoupling of NOS in our study. However, the increase in total nitrite/nitrate levels after arginase inhibition only in preeclamptic group further confirms a higher arginase activity in endothelial cells treated with preeclamptic plasma only.
Since, inhibition of iNOS with 1400W did not significantly reduce superoxide, iNOS is likely not a source of superoxide in response to plasma from women with preeclampsia. However, iNOS inhibition reduced nitrotyrosine staining to greater extent suggesting that iNOS is a source of NO when exposed to plasma from women with preeclampsia. An increased iNOS expression in response to preeclamptic plasma also supports these data. On the other hand, in the presence of preeclamptic plasma, l-arginine supplementation significantly reduced superoxide levels but increased nitrotyrosine staining. The increase in nitrotyrosine staining could have been due to increased NO production partially via an activated eNOS, and also via iNOS. iNOS activity increases linearly with increasing concentrations of extracellular l-arginine.45 Moreover, during high output NO situations, NOHA an intermediate generated during NO synthesis can inhibit arginase.45 This can rescue eNOS from uncoupling, leading to increased NO formation and reduced superoxide. However, superoxide is still being produced by NADPH oxidase that an abundant production during l-arginine supplementation could lead to enhanced peroxynitrite formation as observed in our study.
In the third part of this experiment, we examined superoxide and peroxynitrite levels after inhibition of arginase, NADPH oxidase, or both in the presence or absence of l-arginine supplementation. Here we note that arginase inhibition alone reduced superoxide but increased nitrotyrosine staining as noted in the first part of our experiment. As expected l-arginine did not affect superoxide levels, but increased nitrotyrosine staining possibly due to excess NO formation that reacts with superoxide generated via NADPH oxidase. Interestingly, DPI alone significantly reduced both superoxide and peroxynitrite, and that l-arginine supplementation further reduced superoxide levels but not peroxynitrite. This is likely because, l-arginine supplementation provides substrate for NOS to produce NO and partially prevents uncoupling. However, after inhibition of both arginase and NADPH oxidase, we find that l-arginine supplementation almost abolished superoxide levels and nitrotyrosine staining. l-arginine supplementation in the face of oxidative stress may in fact prove to be harmful due to the formation of peroxynitrite. However, in the presence of an NADPH oxidase inhibitor, a cocktail of increased NO and decreased superoxide resulted in the greatest reduction of nitrotyrosine.
We also examined the effect of increasing concentrations of l-arginine on nitrotyrosine formation in endothelial cells treated with plasma (see Supplementary material online, Figure S1). We observed increased nitrotyrosine formation in all groups, most likely due to excess formation of nitric oxide. However, these responses were maximal at 2 mmol/L. Thus, administration of l-arginine to any group of women in the presence of superoxide formation is likely to result in peroxynitrite formation.
In summary, our study shows that increasing l-arginine availability either by inhibiting arginase or supplementing excess l-arginine in the face of excess oxidative stress may be more damaging, particularly due to formation of peroxynitrite. Therefore, arginase inhibition alone or l-arginine supplementation alone could result in detrimental effects to the endothelium. On the other hand, focusing on inhibiting the oxidant pathway such as inhibition of both arginase and NADPH oxidase simultaneously could be beneficial. After inhibition of the oxidant pathway, further supplementation of l-arginine did not result in excess peroxynitrite formation. Thus this study provides important insights in regard to the use of l-arginine as a therapeutic modality in preeclampsia.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was supported by grants from the Canadian Institutes for Health Research (CIHR). S.S. was supported by the Maternal-Fetal-Newborn Health and Strategic Training Initiative in Research in Reproductive Health Sciences Training Programs of the CIHR, the Alberta Heritage Foundation for Medical Research (AHFMR), and the Junior Personnel Award from the Heart and Stroke Foundation of Canada. H.X. was supported by a Summer Studentship from the AHFMR. S.T.D. is an AHFMR Scientist and a Canada Research Chair in Women's Cardiovascular Health.
Acknowledgements
The authors thank Donna Dawson from the Royal Alexandra Hospital for sample collection.
Conflict of interest: none declared.