-
PDF
- Split View
-
Views
-
Cite
Cite
Yi Tan, Yan Li, Jian Xiao, Hongwei Shao, Chuanlin Ding, Gavin E. Arteel, Keith A. Webster, Jun Yan, Hong Yu, Lu Cai, Xiaokun Li, A novel CXCR4 antagonist derived from human SDF-1β enhances angiogenesis in ischaemic mice, Cardiovascular Research, Volume 82, Issue 3, 1 June 2009, Pages 513–521, https://doi.org/10.1093/cvr/cvp044
Close - Share Icon Share
Abstract
The effects on angiogenesis of a novel CXC chemokine receptor 4 (CXCR4) antagonist, SDF-1βP2G, derived from human stromal cell-derived factor-1β (SDF-1β), were examined in a model of hind limb ischaemia in mice.
The antagonistic activities of SDF-1βP2G against CXCR4 were evaluated in vitro and in vivo and compared with phosphate-buffered saline and AMD3100 (a small bicyclam antagonist of SDF-1). Angiogenesis, muscle regeneration and the expression of pro-angiogenic factors were evaluated in ischaemic gastrocnemius muscles. Distant toxic effects of SDF-1βP2G were evaluated by inflammatory and apoptotic markers. SDF-1βP2G induced CXCR4 internalization and competitively inhibited the chemotaxis of SDF-1β but did not mediate migration, calcium influx, or the phosphorylation of Akt and extracellular signal-regulated kinase in cultured T-lymphoblastic leukaemia cells or H9C2 cells. SDF-1βP2G enhanced blood flow, angiogenesis, and muscle regeneration in ischaemic hind limbs, and the enhancement was significantly better than that of AMD3100. Markers of angiogenesis and progenitor cell migration, including phosphorylated Akt, vascular endothelial growth factor (VEGF), SDF-1 and CXCR4, were up-regulated by SDF-1βP2G and co-localized with CD31-positive cells. Neutralization of VEGF with its specific antibody abolished SDF-1βP2G-induced blood reperfusion and angiogenesis. No apparent inflammatory and apoptotic effects were found in heart, liver, kidneys, and testes after SDF-1βP2G administration.
Our findings indicate that the novel CXCR4 antagonist, SDF-1βP2G, can efficiently enhance ischaemic angiogenesis, blood flow restoration, and muscle regeneration without apparent adverse effects, most likely through a VEGF-dependent pathway.
1. Introduction
Improving angiogenesis and revascularization are therapeutic goals to rescue tissues from critical ischaemia and restore organ function.1 Neoangiogenesis initiates revascularization and recruits endothelial cells that assemble into neovessels.2 Initial trials with the delivery of angiogenic factors, such as vascular endothelial growth factor (VEGF),3 basic fibroblast growth factor,4,5 and stromal cell-derived factor-1 (SDF-1)6–8 to ischaemic muscle have demonstrated improved tissue perfusion in the short term; however, long-term beneficial effects and recovery from ischaemia have not been demonstrated clinically.
The bone marrow contains vascular progenitor cells that can be mobilized to the peripheral circulation and trafficked to ischaemic sites to initiate neoangiogenesis, and assists in revascularization.2 Therefore, increasing the number of pro-angiogenic cells in peripheral blood available to ischaemic sites by mobilization is an attractive therapeutic strategy.9,10 The mobilization of stem cells in the bone marrow is regulated by the local microenvironment, the so-called ‘stem cell niche’, which consists of fibroblasts, osteoblasts, and endothelial cells.11 Mobilizing cytokines interrupt the interactions between cytokines/chemokines on stem cells and/or stromal cells, which then allow stem cells to leave the bone marrow via transendothelial migration.11 Proteinases such as elastase, cathepsin G, and matrix metalloproteinases then cleave adhesive bonds on stromal cells, which interact with integrins on haematopoietic stem cells. The potential of SDF-1 in stem cell mobilization and angiogenesis induction has received extensive attention.9,12 Currently, convincing evidence suggests that local administration of SDF-1 promotes reperfusion and improves angiogenesis through mobilizing and homing endothelial progenitor cells (EPCs) to ischaemic sites.7,8,13 However, there are side effects of SDF-1 and its receptor CXC chemokine receptor 4 (CXCR4) that prohibit the clinical use of SDF-1 for the therapeutic mobilization of pro-angiogenic cells in patients. For instance, SDF-1 traffics not only normal stem cells, but also tumour stem cells that express CXCR4 and may facilitate tumour vasculature development and metastasis.14,15
Acute blockade of the interaction between SDF-1 and CXCR4 by a single dose of CXCR4 antagonists AMD3100 (a small bicyclam molecule inhibitor against SDF-1)16,17 and T-140 (a short modified peptide, also called 4F-benzoyl-TN14003)18 was found to induce a rapid and transient haematopoietic cell mobilization. Short-term administration of AMD3100 showed significant improvement of blood restoration and angiogenesis during acute phase of ischaemia in normal19 and diabetic20 mouse hind limb ischaemic models. But continual administration of AMD3100 failed to persistently improve hind limb blood flow restoration.6 A clinical trial with AMD3100 to inhibit HIV-1 entry was terminated due to safety concerns.21,22 Therefore, there is great interest in developing more specific non-toxic bio-molecules that can antagonize CXCR4 and induce haematopoietic cell mobilization and neoangiogenesis.
N-terminal amino acid residues1–8 in SDF-1α are the major site for direct interaction with the receptor and subsequent signal transduction, whereas the second amino acid proline plays a key role in SDF-1α receptor activation.23,24 Therefore, in the present study, we developed a novel peptide antagonist against CXCR4, SDF-1βP2G, by replacing the N-terminal second proline residue of human SDF-1β with glycine. We found that SDF-1βP2G potently antagonized CXCR4 and significantly improved blood flow reperfusion, angiogenesis, and muscle regeneration in mouse hind limb ischaemic model without apparent adverse effects.
2. Methods
Detail materials and methods are provided by Supplementary material online. In the below section, only the main materials and main steps of the methods are described.
2.1. Cell lines and reagents
MOLT-4 (human acute T-lymphoblastic leukaemia cell line) and H9C2 (rat embryonic cardiac myoblasts) cells were purchased from American Type Culture Collection (Manassas, VA, USA). AMD3100, monoclonal anti-β-actin antibody and Naphthol AS-D Chloroacetate Esterase staining kit were purchased from Sigma (St Louis, MO, USA), rat anti-mouse CD31 antibody was purchased from BD Bioscience (San Jose, CA, USA), phospho-Akt (Ser473) (p-Akt) rabbit monoclonal antibody and Akt rabbit monoclonal antibody were purchased from Cell Signalling Technology (Danvers, MA, USA), VEGF rabbit polyclonal antibody, fusin (G-19) goat polyclonal antibody, SDF-1 rabbit polyclonal antibody, phospho-extracellular signal-regulated kinase 1/2 (p-ERK1/2) rabbit monoclonal antibody, ERK1 rabbit monoclonal antibody were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA), proliferating cell nuclear antigen (PCNA) was purchased from DakoCytomation (Carpinteria, CA, USA), and ApopTag In Situ kit was purchased from Chemicon (Temecula, CA, USA).
2.2. Preparation of recombinant human SDF-1β and SDF-1βP2G
The primers for cloning human SDF-1β and its mutant SDF-1βP2G were designed and synthesized according to the cDNA sequences of native SDF-1β. SDF-1β and SDF-1βP2G cDNA were amplified by reverse transcriptional polymerase chain reaction from human bone marrow total mRNA, and the recombinants pET-30a(+)/SDF-1β and pET-30a(+)/SDF-1βP2G were expressed in BL21 (DE3) system and purified and verified as in previous report.25
2.3. Characterization of the recombinant human SDF-1βP2G
The antagonistic activities of SDF-1βP2G against CXCR4 were evaluated in vitro by CXCR4 internalization, chemotaxis and competitive chemotaxis inhibition. The intracellular effects of SDF-1βP2G were evaluated by calcium influx assays, p-Akt and p-ERK1/2 expression using either MOLT-4 or H9C2 cells, and based on our published methods.25,26
2.4. Hind limb ischaemic mouse model and drug delivery
Male FVB (8 weeks old) were purchased from Jackson Laboratory (Bar Harbor, ME, USA) and maintained under specific pathogen-free conditions at the University of Louisville Animal Facility. The hind limb ischaemic model was performed as described previously.7,8,27 SDF-1βP2G at the concentration of 5 mg/kg body weight was given daily by intravenous injection either beginning 1 day before surgery (subgroup I) or 3 h after surgery (subgroup II) until day 14 post-surgery (Supplementary material online). AMD3100 at 5 mg/kg body weight (subcutaneously) and phosphate buffered saline (PBS) (intravenously) were injected as the positive and negative controls, respectively. Because AMD3100 has been extensively used at this level, 5 mg/kg body weight has been selected for both SDF-1βP2G and AMD3100.16,17,19,20 Additional group of mice were given SDF-1βP2G (5 mg/kg body weight) immediately after surgery, along with intraperitoneal injection of neutralizing anti-mouse VEGF mAb (R&D Systems Inc., Minneapolis, MN, USA) at a final concentration of 400 µg/kg body weight in 100 µL PBS every 3 days. The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication no. 85–23, revised 1996). All experiments were approved by the Animal Care and Use Committee of the University of Louisville.
2.5. Laser Doppler perfusion images
Limb blood flow was monitored using a laser Doppler perfusion imager (LDPI) as described previously.7,8
2.6. Histological analysis and capillary density
Paraffin sections (5 µm) from ischaemic gastrocnemius muscles dissected from mice on day 14 after surgery were stained with haematoxylin and eosin and observed under light microscopy.
Cryostat sections (5 µm) from OCT-embedded tissue samples of the ischaemic gastrocnemius muscles dissected from mice at different time points after surgery were stained with rat anti-mouse CD31 (1:50) antibody, counterstained with haematoxylin, and observed under light microscopy. Detail quantitative analysis is provided in Supplementary material online. Capillary density is expressed as number of capillaries per muscle fibre and includes necrotic fibres where identified.
2.7. Immunofluorescent staining and western blotting
Cryostat sections (5 µm) from ischaemic gastrocnemius muscles dissected from mice on day 7 after surgery were co-incubated with rat anti-mouse CD31 and p-Akt rabbit monoclonal antibody, VEGF rabbit polyclonal antibody, fusin (G-19) goat polyclonal antibody, or SDF-1 rabbit polyclonal antibody, respectively. The expression of different proteins was quantified by fluorescence microscopy. P-Akt, total Akt, p-ERK1/2, ERK1, and VEGF were also quantified by western blotting assay.28
2.8. Analysis of cell proliferation, apoptosis, and inflammatory response
Cell proliferation in gastrocnemius muscle was detected with immunohistochemical staining of PCNA (1:50 dilution). The apoptotic and inflammatory responses in the heart, liver, kidney, and testis were examined with an ApopTag In Situ kit and Naphthol AS-D Chloroacetate Esterase staining kit according to the respective manufacturers recommendations.
2.9. Statistical analysis
All data are presented as mean ± SE. Statistical analysis was performed using Origin 7.5 (OriginLab data analysis and graphing software) with one-way or two-way ANOVA, followed by post-multiple comparisons with the Scheffe’ test. Statistical significance was considered at P < 0.05.
3. Results
3.1. Preparation of recombinant human SDF-1β and SDF-1βP2G in Escherichia coli expression system
Both native SDF-1β and SDF-1βP2G were efficiently expressed in inclusion bodies in pET-30a(+)-BL21 (DE3) bacterial expression system (Supplementary material online, Figure S1A). The recombinant proteins were purified by Ni2+ affinity chromatography and refolded under optimal conditions (Supplementary material online, Figure S1B). The N-terminal 6×His-Tag was digested by enterokinase (Supplementary material online, Figure S1C and D) and separated by RP-HPLC (Supplementary material online, Figure S1E and F). The N-terminal amino acid sequences were confirmed by peptide sequencing (data not shown).
3.2. Characterization of SDF-1βP2G
Experiments were implemented to determine whether the physical and functional interactions between SDF-1βP2G and CXCR4 were preserved. First, since SDF-1-induced CXCR4 internalization is a functional marker of this interaction,29 we treated MOLT-4 cells with SDF-1β or SDF-1βP2G under the indicated conditions and measured the amount of surface CXCR4 by flow cytometry. SDF-1βP2G induced CXCR4 internalization in a dose-dependent manner, with 98% internalization at a dose of 1 µmol/L, similar to the dose–response of native SDF-1β (Supplementary material online, Figure S2A). Migration of CXCR4+T-lymphocytes is another functional marker of native SDF-1.25,26 Therefore we tested for this activity. As shown in Supplementary material online, Figure S2B, native SDF-1β, but not SDF-1βP2G, induced a dose-dependent chemotaxic effect within the dose range from 0.01 to 1.0 nM (P < 0.01). The effectiveness of this response decreased at higher doses. Secondly, we determined whether pre-bound SDF-1βP2G prevented the subsequent binding of native SDF-1β to CXCR4 thereby blocking SDF-1β-chemotaxis. MOLT-4 cells were pre-incubated with increasing concentrations of SDF-1βP2G for 2 h and then subjected to a chemotaxic assay with 1 nmol/L of SDF-1β. SDF-1β-induced cell migration was abolished by pre-incubation with SDF-1βP2G in a dose-dependent manner (Supplementary material online, Figure S2C).
Cell activation by the binding of SDF-1 to CXCR4 involves an increase in intracellular-free calcium.25,26 We confirmed a rapid, transient increase of intracellular calcium levels when cells were exposed to SDF-1β, but not to SDF-1βP2G (Supplementary material online, Figure S2D). Therefore, CXCR4 is activated on these cells by SDF-1β but not by SDF-1βP2G. To confirm the absence of CXCR4 activation by SDF-1βP2G at the level of intracellular signalling, we measured Akt and ERK phosphorylation and VEGF expression in H9C2 cells after treatments with SDF-1β or SDF-1βP2G (Supplementary material online, Figure S3A). Despite there was significantly dose-dependent increases of p-Akt and p-ERK1/2 in response to SDF-1β, there was no significant effect of SDF-1βP2G. Total protein levels were unchanged in both cases (Supplementary material online, Figure S3A–C). These experiments indicate that SDF-1βP2G binds to CXCR4 and induces internalization, but does not trigger cell migration, intracellular calcium influx, or the associated-signalling pathways.
3.3. SDF-1βP2G improvement of the blood reperfusion and angiogenesis in mouse ischaemic hind limb
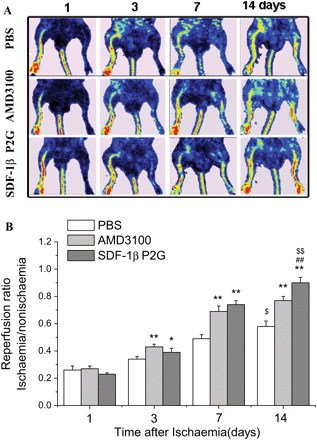
Ischaemia was induced in the mouse hind limb as described in Methods section. Return of blood flow to the ischaemic hind limb was monitored by LDPI imager (Figure 1A) and quantified by LDPIwin2.5 software. On day 3 and 7 after ischaemia, both SDF-1βP2G and ADM3100 induced a significant increase of hind limb blood reperfusion compared with PBS treatment. On day 14, AMD3100 did not further increase the blood reperfusion relative to day 7; however, the SDF-1βP2G group demonstrated progressive and significantly increased reperfusion at day 7 and 14, (P < 0.01, Figure 1B). Therefore, AMD3100 induced only short-term, recovery of blood flow, but this was significantly extended by SDF-1βP2G treatment.
SDF-1βP2G improved blood flow restoration in mouse ischaemic hind limb. The limb blood flow was measured using a laser Doppler perfusion imager (LDPI) and quantified using LDPIwin 2.5 software. (A) Representative laser Doppler perfusion colour images for different groups. (B) To minimize the variability in perfusion, the ratio of the ischaemic (left) to normal (right) limb blood flow was used for quantitative analysis (n = 13; for each experimental group). *P < 0.05, **P < 0.01 vs. PBS control; ##P < 0.01 vs. AMD3100; $P < 0.05, $$P < 0.01 vs. group of day 7.
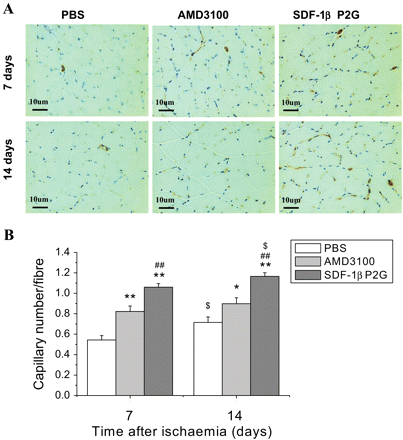
Capillary density in the ischaemic hind limb gastrocnemius muscle was assessed by immunohistochemical staining with anti-mouse CD31 antibody (Figure 2A). The density of capillaries in mice treated with SDF-1βP2G was significantly higher than that in mice treated with PBS or AMD3100 on both day 7 and 14 after ischaemia (P < 0.05, Figure 2B). There was no significant difference in capillary density for AMD3100 treatment between day 7 and 14 (P > 0.05, Figure 2B), whereas the capillary density on day 14 for SDF-1βP2G was significantly higher than that of day 7 (P < 0.05, Figure 2B). These results indicate that the increased blood reperfusion in ischaemic hind limb by SDF-1βP2G and AMD3100 correlates with increased angiogenesis, and SDF-1βP2G but not AMD3100 mediates a persistent angiogenic stimulus.
SDF-1βP2G improved angiogenesis in gastrocnemius muscle in mouse ischaemic hind limb. Capillaries (brown dots) were identified by CD31 staining (A) and quantitatively expressed as a capillary number per muscle fibre on 7 and 14 days after ischaemic operation (n = 15; for each experimental group). *P < 0.05, **P < 0.01 vs. PBS control; ##P < 0.01 vs. AMD3100; $P < 0.05 vs. group of day 7.
3.4. SDF-1βP2G enhancement of muscle regeneration in ischaemic hind limb
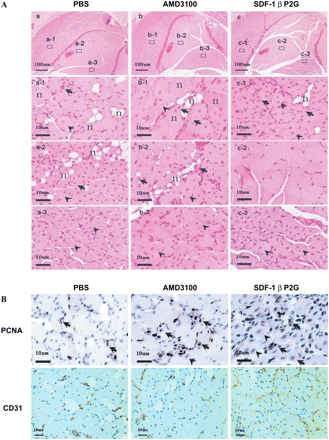
We next examined the histopathology of gastrocnemius muscle in the ischaemic hind limbs. Representative morphological changes are shown in Figure 3A. Pathological examination found that muscles in the PBS-treated group were largely necrotic (Figure 3A, a-1 and a-2, n) with minimal evidence of proliferation and regeneration (Figure 3A, a-1 and a-2, arrows and arrowheads). Similarly, necrotic fibres were highly evident in the muscles of AMD3100-treated mice (Figure 3A, b-1 and b-2, n), proliferating myoblasts were rare and there was sparse evidence of regenerating muscle with central nuclei (Figure 3A, b-1 and b-2, arrows; Figure 3A, b-1 and b-2, arrowheads). In contrast, in the SDF-1βP2G-treatment group, there was extensive proliferation of myoblasts (Figure 3A, c-1, arrows) and regenerating muscles with central nuclei (Figure 3A, c-1, arrowheads), limited numbers of necrotized myofibres (Figure 3A, c-1, n), and predominantly undamaged muscles (Figure 3A, c-2). These features suggest an early absorption of the necrotic muscle and regeneration of new muscle probably due to the timely return of blood flow and angiogenesis promoted by SDF-1βP2G.
SDF-1βP2G-induced muscle regeneration in mouse ischaemic hind limb. (A) Histological examination by haematoxylin and eosin (H&E) staining for the proximal portion of gastrocnemius muscle of the ischaemic hind limb from various groups of mice on day 14 after ischaemic surgery. Low-power views were presented in the top row, and high-power views for different areas indicated by numbers in the top row were presented in rest rows to reveal the typical pathological changes. (B) Immunohistochemical staining for PNCA and CD31 in regenerating areas of gastrocnemius muscle. Arrows, proliferating myoblasts; arrow heads, regenerating muscles with central nuclei; n, nectrotic fibre.
It should be mentioned that the muscle fibres in the regions near fibula showed strong regeneration in all three groups (Figure 3A, a-3, b-3, and c-3, arrowheads). This may suggest that the compensative blood supplementation in the regions near the fibula is more efficient than other regions in gastrocnemius muscles.
Regenerating areas were stained with PCNA and CD31 antibodies for the examination of cell proliferation, tissue angiogenesis, and neovascular generation. In SDF-1βP2G-treated group, PCNA positive-staining muscles with central nuclei (Figure 3B, arrowheads) and myoblasts (Figure 3B, arrows) were extensive, and the regenerating muscles surrounded by abundant new vessels (Figure 3B) were predominant. In contrast, in AMD3100- and PBS-treated groups, these findings were relatively rare (Figure 3B, arrows and arrowheads), and the debris and necrotized muscle fibres were also rarely surrounded by neovessels (Figure 3B).
3.5. SDF-1βP2G up-regulation of pro-angiogenic factors
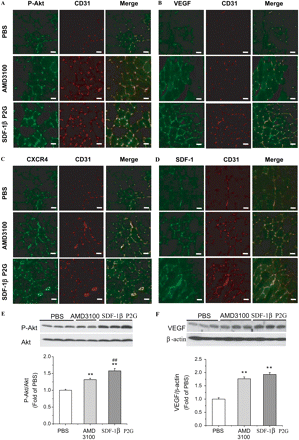
To address mechanism and quantify signalling intermediates under the different stimuli, we measured p-Akt, VEGF, CXCR4, and SDF-1 by immunohistochemistry co-stained with CD31 and by western blotting (Figure 4). On day 7 after ischaemia, p-Akt and VEGF co-localized with CD31-positive cells (Figure 4A and B) and were significantly up-regulated in the SDF-1βP2G-treated group compared with PBS (Figure 4E and F). p-Akt and VEGF were also significantly up-regulated on day 7 in the AMD3100 treatments, but the inductions were relatively lower than that of SDF-1βP2G-treated group (Figure 4E and F). Interestingly, SDF-1 and CXCR4 were also up-regulated and co-localized with CD31 similar to p-Akt and VEGF (Figure 4C and D). These results support a mechanism of revascularization in this model whereby Akt and VEGF promote angiogenesis, and here is an early recruitment of CXCR4-positive cells to ischaemic muscle. These events are significantly enhanced by SDF-1βP2G but less so by AMD3100.
Pro-angiogenic factors co-localized with CD31 in gastrocnemius muscle in mouse ischaemic hind limb. Expression of p-Akt (A), VEGF (B), CXCR4 (C), and SDF-1 (D) (green) was respectively co-stained with CD31 (red) in the cryostat sections from tissues dissected from mice at 7 days after surgery using proper antibodies and examined under fluorescent confocal microscopy (bar = 10 µm). The expression of total Akt and p-Akt (E), and VEGF protein (F) in ischaemic muscle on day 7 after ischaemia was quantified by western blotting assay. Akt activation and VEGF expression were expressed by ratios of P-Akt/Akt and VEGF/β-actin, respectively (n = 11; for each experimental group). **P < 0.01 vs. PBS; ##P < 0.01 vs. AMD3100.
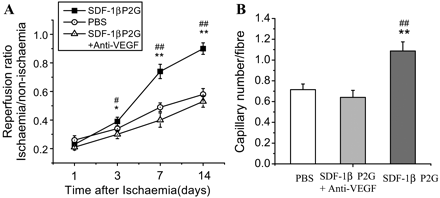
To further define the role of VEGF in SDF-1βP2G-induced revascularization, we treated ischaemic hind limbs with SDF-1βP2G and a neutralizing anti-VEGF mAb. As shown in Figure 5A and B, the enhanced blood perfusion and capillary density caused by SDF-1βP2G were both attenuated back to the level of the PBS group by anti-VEGF antibody. These results indicate that VEGF is an essential pathway component of SDF-1βP2G-induced angiogenesis and is required for the enhanced restoration of blood flow.
Impact of neutralizing anti-VEGF mAb on SDF-1βP2G-induced ischaemic blood reperfusion and angiogenesis. Mice were treated with either SDF-1βP2G+anti-VEGF mAb, SDF-1βP2G, or PBS after ischaemia (n = 7). The limb blood flow was monitored by a laser Doppler perfusion imager (LDPI) on day 1, 3, 7, and 14 after ischaemia (A), and the capillaries were identified by CD31 staining on day 14 after ischaemia (B) (n = 7; for each experimental group). *P < 0.05, **P < 0.01 vs. PBS; #P < 0.05, ##P < 0.01 vs. anti-VEGF mAb treatment.
3.6. Apoptosis and inflammation
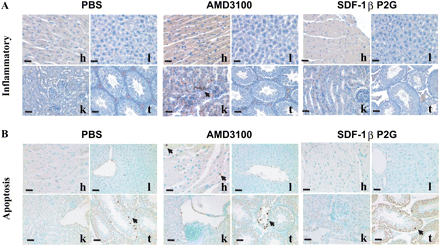
To evaluate possible distant toxicity of SDF-1βP2G and AMD3100, we examined inflammation and apoptotic indices in heart, liver, kidneys, and testes on day 14 after ischaemia using Naphthol AS-D Chloroacetate Esterase and TUNEL staining. We found a few inflammatory cells infiltrated in the kidney and apoptotic cells in the heart of mice treated with AMD3100, but no obvious inflammatory effects and apoptosis in the heart, liver, and kidney of mice treated with SDF-1bP2G compared with PBS treatment (Figure 6A and B).
The organic toxicity of SDF-1βP2G and AMD3100 on day 14 after ischaemia. (A) Cell inflammatory reactions were examined by specific Naphthol AS-D Chloroacetate Esterase staining, bright red granulation showing the granulocytic lineage inflammatory cells. (B) Apoptotic cells were detected by transferase-mediated dUTP nick-end labelling (TUNEL) staining (cells with brown dots). h, heart; l, liver; k, kidney; and t, testis (bar = 10 µm).
4. Discussion
We have shown that SDF-1βP2G, a CXCR4 antagonist derived from human SDF-1β, improves blood flow, angiogenesis, and muscle regeneration in a hind limb ischaemic mouse model. Unlike the previously tested CXCR4 antagonist AMD3100, SDF-1βP2G provided a persistent recovery of hind limb perfusion, along with the activation of Akt and up-regulation of VEGF.
Stimulation of blood restoration and angiogenesis after hind limb ischaemia by mobilizing stem cells into peripheral blood and ischaemic injury tissues has been extensively investigated.7,8,30–37 These studies provided new insight into the mechanisms underlying how stem cells are homing to ischaemic tissues and initiating the neoangiogenesis and revascularization processes. It is appreciated now that for the mobilized stem cells from bone marrow, trafficking them to ischaemic tissue is the critical step to successfully initiate the neoangiogenesis and revascularigenesis.1,6–8,19,35,38 Up-regulation of several molecules, including SDF-1, CXCR4, and VEGF expression in the ischaemic tissues was found to play critical roles in recruiting these stem cells to ischaemic tissues.39–41 In the present study, we found that SDF-1 and CXCR4 expressions were obviously higher in SDF-1βP2G group than that in AMD3100 and PBS groups (Figure 4C and D). Recently Hur et al.32 demonstrated the importance of Akt expression in the local tissues for the stem cells trafficking to ischaemic area. They found that Akt was activated in endothelial cells of ischaemic muscle by ischaemia-induced VEGF and SDF-1, and the activated Akt was found to enhance intercellular adhesion molecule 1 expression on endothelial cells, which is a key step for stem cells stick on ischaemic endothelial cells. In support of this important finding, we found that SDF-1βP2G significantly enhanced VEGF expression (Figure 4B and F) along with up-regulated Akt phosphorylation (Figure 4A and E) in the ischaemic tissues. Neutralization of VEGF completely prevented SDF-1βP2G-induced hind limb blood reperfusion and angiogenesis (Figure 5), suggesting that the up-regulation of VEGF expression in ischaemic tissue is directly involved in SDF-1βP2G-induced ischaemic blood perfusion and angiogenesis. The up-regulation of VEGF in the ischaemic tissues may be indirectly mediated by SDF-1βP2G, since it does not directly stimulate VEGF expression and Akt and ERK1/2 phosphorylation in the in vitro conditions (Supplementary material online, Figure S3).
AMD3100, another commonly used CXCR4 potent antagonist, has been proven to improve acute phase blood reperfusion and angiogenesis within 7 days after ischaemic operation in normal and diabetic mice hind limb ischaemic injury.19,20 However, chronic administration of AMD3100 (twice daily for 21 days) failed to improve ischaemic hind limb blood perfusion and function restoration.6 The present study also supports the improvement of AMD3100 for blood restoration and angiogenesis within acute phase post-ischaemia, without significant persistent benefits (Figures 1B and 2B). When compared with AMD3100, SDF-1βP2G offers a persistent improvement for hind limb blood reperfusion and angiogenesis (the present study), even though we have not identified the mechanism for this difference. The effects of SDF-1βP2G on the hind limb were similar to those of SDF-1, where a persistent improvement of blood reperfusion and angiogenesis has also been demonstrated.7,8,13 However, the mechanism of action of SDF-1βP2G and SDF-1 are clearly different. Native SDF-1 works by directly attracting EPCs to ischaemic sites,7,8,13 whereas the effects of SDF-1βP2G are indirect.
For any newly developed medication, the safety is always a concern. Therefore, we have comparatively evaluated the potential toxic effects on heart, liver, kidneys, and testes after mice were treated with SDF-1βP2G and AMD3100 for 14 days by pathological examination. We found a few inflammatory cells infiltrated in the kidney and apoptotic cells in the heart of mice treated with AMD3100, but no obvious inflammatory effects and apoptosis in the heart, liver, and kidney of mice treated with SDF-1βP2G compared with PBS treatment (Figure 6A and B).
A limitation of the present study is the lack of a dose–response study on both SDF-1βP2G and AMD3100, which can provide a more detail comparison between two antagonists. In addition, less apoptotic effect of SDF-1βP2G when compared with AMD3100 can be considered as a less toxic effect for normal tissues; however, whether this less apoptotic effect of the SDF-1βP2G will contribute to tumour genesis remains to be systemically evaluated in future before it can be considered for clinical application.
In summary, we developed a novel CXCR4 antagonist, SDF-1βP2G that was derived from human SDF-1β. The SDF-1βP2G has a potent antagonistic action against CXCR4 reaction with its native ligand SDF-1β and also significantly improved the limb blood flow, angiogenesis, and muscle regeneration timely and efficiently in hind limb ischaemic mouse model without inflammatory and apoptotic effects in examined organs. We propose that SDF-1βP2G may be a safe approach for the treatment of peripheral ischaemia.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was supported in part by research grants from Natural Science Foundation (30600768, 2006A099, Y206644, and QTJ05013 to Y.T.); Program of New Century Excellent Talents in University and Zhejiang Provincial Program for the Cultivation of High-level Innovative Health Talents in China (706018 to X.L.); American Diabetes Association (02-07-JFA-10; 05-07-CD-02 to L.C.); Juvenile Diabetes Research Foundation (5-2006-382 to X.L. and L.C.); Wenzhou Medical College Start-Up Fund for the Chinese-American Research Institute for Diabetic Complications (to L.C. and X.L.); and National Institutes of Health (HL 076356 to H.Y. and HL 072924 to K.A.W.).
Acknowledgements
We thank Jingwen Zhang (University of Louisville School of Medicine) for technical assistance with the hind limb ischaemic surgery.
Conflict of interest: none declared.
References
Author notes
The first two authors contributed equally to the study.