-
PDF
- Split View
-
Views
-
Cite
Cite
Willem M.H Hoogaars, Alessandra Tessari, Antoon F.M Moorman, Piet A.J de Boer, Jaco Hagoort, Alexandre T Soufan, Marina Campione, Vincent M Christoffels, The transcriptional repressor Tbx3 delineates the developing central conduction system of the heart, Cardiovascular Research, Volume 62, Issue 3, June 2004, Pages 489–499, https://doi.org/10.1016/j.cardiores.2004.01.030
Close - Share Icon Share
Abstract
Objective: The molecular mechanisms that regulate the formation of the conduction system are poorly understood. We studied the developmental expression pattern and functional aspects of the T-box transcription factor Tbx3, a novel marker for the murine central conduction system (CCS). Methods: The patterns of expression of Tbx3, and of Cx40, Cx43, and Nppa, which are markers for atrial and ventricular chamber-type myocardium in the developing heart, were analyzed in mice by in situ hybridization and three-dimensional reconstruction analysis. The function of Tbx3 in regulating Nppa and Cx40 promoter activity was studied in vitro. Results: In the formed heart, Tbx3 is expressed in the sinoatrial node (SAN), atrioventricular node (AVN), bundle and proximal bundle branches (BBs), as well as the internodal regions and the atrioventricular region. Throughout cardiac development, Tbx3 is expressed in an uninterrupted myocardial domain that extends from the sinoatrial node to the atrioventricular region. This expression domain is present in the looping heart tube from E8.5 onwards. Expression of the chamber-type myocardial markers is specifically absent from the Tbx3 expression domain. Tbx3 is able to repress Nppa and Cx40 promoter activity and abolish the synergistic activation of the Nppa promoter by Tbx5 and Nkx2.5. Conclusion: We identified the T-box transcription factor Tbx3 as a novel and accurate marker for the central conduction system. Our analysis implicates a role for Tbx3 in repressing a chamber-specific program of gene expression in regions from which the components of the central conduction system are subsequently formed.
1. Introduction
The cardiac conduction system is responsible for setting up and maintaining the coordinated rhythmic excitation of the mature heart. In the embryonic and fetal heart, the conduction system can be divided into two component parts: (1) the central conduction system (CCS), comprising the sinoatrial node (SAN), atrioventricular junction including the atrioventricular node (AVN), and the interventricular (IV) ring including its derivatives, the retroaortic root branch, right atrioventricular ring bundle, atrioventricular bundle (AVB), and proximal part of the bundle branches (BBs); and (2) the peripheral conduction system encompassing the distal part of the BBs plus the peripheral ventricular conduction network (PVCN) [1,2]. From early stages of development onwards, the PVCN, like the atrial and ventricular chambers, is characterized by fast conduction [3] and expression of chamber-specific genes such as natriuretic precursor peptide A (Nppa) and gap junction genes connexin40 (Cx40) and connexin43 (Cx43) [4–6]. In contrast, the CCS components are characterized by slow conduction and lack of expression of these chamber-specific genes [4–6]. At late fetal stages, the proximal BB and AVB develop high conduction velocities and initiate the expression of Cx40[4,7]. Our current understanding of the mechanisms underlying the formation of the CCS is poor, in part because of the very limited availability of good markers [1–3,6,8,9].
We recently found that the CCS components develop within the expression domain of T-box transcription factor Tbx2. Tbx2 represses chamber-specific genes Nppa and Cx40 and blocks chamber formation [10,11]. Therefore, the CCS components develop within a region in which chamber formation is suppressed. From early fetal stages, cardiac expression of Tbx2 decreases. However, the cardiac expression of Tbx3 increases. Tbx3 and Tbx2 share structure, repressor function, and DNA recognition elements [12–14]. In this study, we show by quantitative three-dimensional reconstruction that throughout development, Tbx3 is selectively expressed in the entire CCS. Furthermore, Tbx3 was found to repress the promoter activity of Nppa and Cx40. Our results indicate that Tbx3 is involved in the continuous repression of chamber phenotype development in the myocardial regions from which the CCS components form.
2. Methods
2.1. Cell culture and transient transfections
A 1.2-kbp mouse Cx40 upstream regulatory region, from −1196 to +62 relative to the transcription start site, was obtained by polymerase chain reaction (PCR) using FVB mouse genomic DNA and was inserted in pGL3 basic (Promega) for transient transfections. The Nppa-700-luc construct was kindly provided by M. Nemer (Montreal, Canada). Cos-7 cells were cultured and transfected as described [10]. Furthermore, 200 ng of CMV-lacZ was transfected as an internal control. Cells were harvested 48 h after transfection, and luciferase and β-galactosidase activities were assessed. Results shown are from one representative experiment out of at least three done in duplicate. The error bars indicate the difference between the duplicates.
2.2. Electromobility shift assays and Western blot analysis
Nuclear extracts were prepared from HEK cells cultured in 10-cm dishes (1 × 106 cells/dish) transfected with 40 μg of expression constructs. EMSA was performed as described previously [10]. Oligonucleotides used were: TN (corresponding to the TBE-NKE site at position −250 in the Nppa promoter), 5′-TCTGCTCTTCTCACACCTTTGAAGTGGGGGCCTCTTG and TmutN, 5′-TCTGCTCTTCTCTTTGCTTTGAAGTGGGGGCCTCTTG (mutated T-box site underlined). Western blot analysis of nuclear extracts was performed according to standard procedures. Primary antibodies used were anti-HA mAb-HRP (1:1000; Roche) or anti-FLAG rabbit IgG (1:700; ABR) with donkey anti-rabbit IgG-HRP (1:5000; Amersham). The complexes were visualized using the ECL detection kit (Amersham).
2.3. In situ hybridization and three-dimensional reconstruction
Staged mouse embryos obtained from timed mated FVB mice were fixed overnight in 4% paraformaldehyde in phosphate-buffered saline (PBS) and processed for nonradioactive section in situ hybridization, performed as described [15]. The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). RNA probes complementary to the mRNAs for Nppa, chicken Nppa, Cx40, Cx43, and myosin heavy chain (MHC) ATP binding site, which recognizes all MHCs [16], mouse Tbx5 [17], mouse and chicken Tbx3 [17,18], and human TBX3 [14], were labeled with digoxigenin UTP and hybridized to 10- to 14-μm-thick sections. Three-dimensional visualization and geometry reconstruction of patterns of gene expression have been performed as described [15].
3. Results
3.1. Onset and conservation of Tbx3 expression
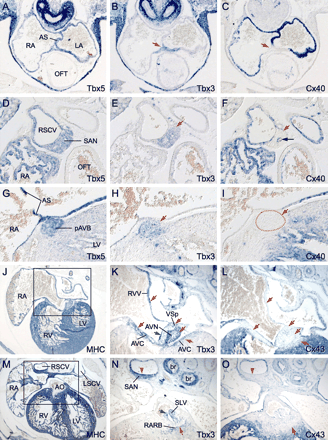
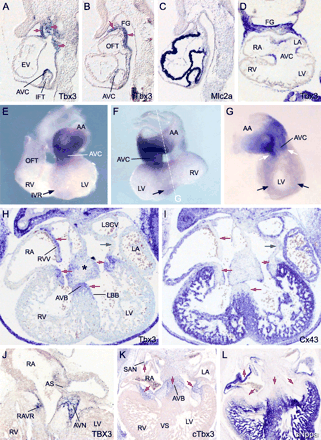
Myocardial Tbx3 expression could first be observed in the IFT and future AVC region of the tubular heart at E8.5 and became stronger at around E9 (Fig. 1A–C). Expression was also observed in the pharyncheal ectoderm, mesoderm, including the dorsal pericardial mesoderm, and in the epithelium of the foregut. At E9.5, expression was clearly confined to the AVC and dorsal side of the embryonic atrium close to the IFT or sinus horns (Fig. 1D–G). In addition, a ring of Tbx3-positive cells was visible around the IV foramen (Fig. 1E–G), which includes the top of the IV septum where the AVB develops, thus identifying the ‘IV ring’ [19,20]. The ring is contiguous with the expression domain in the AVC (Fig. 1G). From E12.5 onwards, staining protruded slightly into the flanks of the IV septum in the direction of the future bundle branches. The AVC and OFT cushion mesenchyme also expressed Tbx3, the highest expression levels being at the side of the lumen (Fig. 1H). In older embryos, expression was additionally observed in the forming AV and OFT valves (Fig. 2K and N). The patterns of Tbx3 expression in a prototypical fetal human heart and chicken heart, in which both the chambers and the CCS components are present, were essentially the same when compared to the pattern of Tbx3 in the fetal mouse heart (Fig. 1H, J, and K), and included the region where the SAN, entire AVC, and AVB develop. These conserved patterns suggest conservation of Tbx3 function.
Expression profile of Tbx3 in the central conduction system precursors in relation to expression patterns of Tbx5, Cx40, and Cx43. All sections are hybridized with probes as indicated. Red arrows indicate regions of reciprocal Tbx3 and Cx40/Cx43 expression. (A–C) Serial sections of an E11.5 mouse heart. (D–H) Serial sections of an E17.5 mouse heart. Black arrow in (F) indicates the Cx40-expressing endothelium of the intranodal blood vessel. Dashed line in panel I demarcates the proximal atrioventricular bundle (pAVB) close to the node. (J–O) Serial sections of an E14.5 mouse heart. Note expression of Tbx3 in the vestibular spine (VSp), the forming tricuspid valve (green arrow in K) and semilunar valve (SLV) of the aorta (AO) (green arrow in N) and retroaortic root branch (RARB). br, bronchus (see legends to Fig. 1 for abbreviations).
Spatial and developmental pattern of Tbx3 in mouse, and conservation of expression in human and chicken. All sections are hybridized with probes as indicated. (A–C) Serial sagittal sections of an E8.75 mouse heart. Arrows indicate expression of Tbx3 in the atrioventricular canal (AVC), pericardial mesoderm and pharyncheal arch ectoderm, mesoderm, and endoderm. (D) Section of an E9.5 embryonic heart. (E–G) Whole mount hybridized hearts of an E9.5 embryo. Black arrows depict the IV ring; white arrows depict the continuity with the AVC expression domain. Panel G shows a right-side view after removal of the right ventricle (plane of dissection indicated in F). (H–I) Serial sections of an E12.5 heart. Red arrows indicate complementary expression of Tbx3 and Cx43, green arrows indicate a region expressing neither gene. Here we found expression of Tbx2. Tbx3 expression is also detected in the cardiac cushions (*). (J) Section of a 6-week human embryonic heart. (K and L) Serial sections of an HH stage 30 chicken heart. Red arrows indicate complementary expression of cTbx3 and cNppa. AA, atrial appendages; AS, atrial septum; AVN, atrioventricular node; AVB, atrioventricular bundle; SAN, sinoatrial node; FG, foregut; LBB, left bundle branch; RAVR, right atrioventricular ring bundle; L/RA, left/right atrium; L/RV, left/right ventricle; OFT, outflow tract; L/RSCV, left/right superior caval vein; VS, ventricular septum; RVV, right venous valve.
3.2. Complementary expression of Tbx3 and chamber-specific genes within the cardiac Tbx5 expression domain
We next analyzed the pattern of Tbx3 in relation to a set of chamber-specific genes, the major cardiac gap junction genes Cx40 and Cx43, and Nppa, in serial sections of subsequent stages of mouse development (Figs. 1 and 2). Analysis of the pattern of Tbx5 was included because this factor is required for expression of Cx40 and Nppa[21]. Furthermore, Tbx5 and Tbx3 recognize the same DNA binding site and therefore probably compete in the regulation of gene expression.
At all stages examined (E8.75–E17.5), Tbx5 expression was observed in a broad pattern in the heart as previously reported [22]. The pattern includes all the components of the CCS, thereby fully overlapping the myocardial Tbx3 expression domain (Fig. 2 and data not shown). The expression domains of Cx40 and Nppa were restricted to that of Tbx5, underlining the finding that Cx40 and Nppa depend on Tbx5 for their expression. The pattern of Cx43 was much broader in the ventricles than the Tbx5 expression domain, which is confined to the LV trabecules and a small portion of the RV (Figs. 1I and 2L and O and data not shown). Therefore, in contrast to Cx40 and Nppa, expression of Cx43, at least in the ventricles, may not be dependent on Tbx5.
The patterns of Tbx3 and the four chamber genes were found to be mutually exclusive throughout all stages of development (E9–E17.5). Thus, expression of Cx40, Cx43, or Nppa (Figs. 1I and 2, data and not shown) was not found in the Tbx3-expressing parts of the IFT and venous valves, in the AVC, and the IV ring. Also in humans and chickens, expression of the Nppa gene was absent from Tbx3-expressing myocardial areas (Fig. 1L and data not shown). We noted one exception in the mutual exclusive expression patterns. At E15.5, weak Tbx3 expression protruded further into the proximal BB (see below), which by then also expresses Cx40 (data not shown) [4,7]. At E17.5, when the heart has reached its mature form, we observed low levels of Cx40 expression in the AVB (data not shown) [7], coexpressed with Tbx3.
3.3 Tbx3 binds to the Nppa T-box binding site and represses Nppa and Cx40 promoter activity
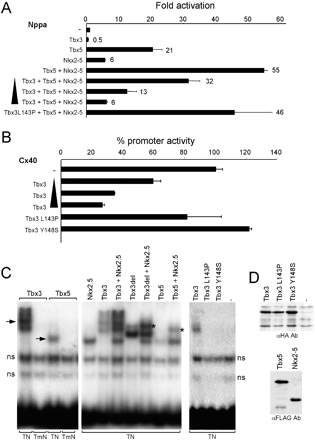
In transgenic mice, the 0.7-kbp rat Nppa promoter drives expression of a reporter gene in the chambers but not in the CCS or its precursors such as the AVC (Ref. [10] and unpublished observations). We next investigated the ability of Tbx3 to suppress this Nppa promoter fragment in the presence of Tbx5 and Nkx2–5, which, like Tbx3, are also expressed in the CCS. As expected, Tbx5 and Nkx2.5 synergistically activated the Nppa promoter (Fig. 3A). When Tbx3 was added, the activation was readily suppressed, indicating that Tbx3 is able to counteract the positive regulation of Tbx5 and Nkx2.5. Tbx3 isoforms with mutations in the T-box that have been found in ulnary–mammary syndrome patients [23], Tbx3L143P, and Tbx3Y149S were unable to interfere with the Tbx5–Nkx2.5-stimulated Nppa promoter activity. The Nppa promoter was repressed only twofold by Tbx3 alone, whereas the Cx40 promoter was repressed fourfold (Fig. 3B).
Tbx3 represses the activity of the promoters of Nppa and Cx40. (A) Tbx3 dose-dependently (50–400 ng) represses the Nppa promoter (4 μg) synergistically activated by Nkx2.5 and Tbx5. Tbx3L143P is unable to repress the activated Nppa promoter. (B) Tbx3 dose-dependently (10–400 ng) represses the Cx40 promoter (4 μg). Tbx3L143P or Y148S does not repress the Cx40 promoter. Fold induction of the activity of the Nppa promoter is given. (C) Electromobility shift assays with nuclear extracts of HEK cells transfected with indicated expression vectors, using the Nppa TBE–NKE module (TN) as a probe. Probe TmN has an inactivating mutation in the T-box binding site. ns, nonspecific bands. Arrows indicate the Tbx3- and Tbx5-specific bands. Ternary complexes are indicated (*). (D) Western blot analysis of nuclear extracts of transfected HEK cells showing HA-tagged wild-type and mutant Tbx3, which are equally well expressed, and FLAG-tagged Nkx2.5 and Tbx5.
We next assessed the ability of Tbx3 to bind to the regulatory module of Nppa that contains a T-box binding site and Nkx factor binding site crucial for repression in the AVC and OFT in vivo [10]. As reported previously [21,24], Tbx5 and Nkx2.5 form a ternary complex (Fig. 3C). Tbx3 is also able to bind to the module with an apparent comparable strength compared to Tbx5 and forms a ternary complex with Nkx2.5. Because of the large and complicated band shift pattern of full-length Tbx3, its carboxy terminal portion (aa 563–723) was deleted to obtain a smaller protein (Tbx3del), which retains its binding capacity but forms a better visible complex with Nkx2.5 on the DNA. Tbx3L143P and Tbx3Y149S were unable to bind the module (Fig. 3C and D) and to form a complex with Nkx2.5 (not shown), indicating that DNA binding of Tbx3 is essential for complex formation.
3.4. Three-dimensional reconstructions of the Tbx3 expression patterns in the developing heart
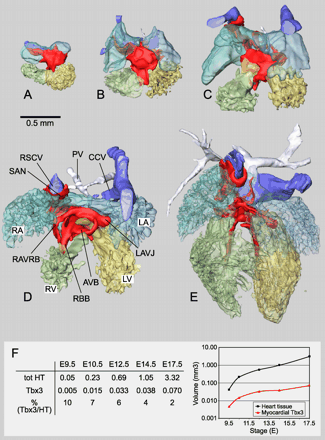
To define the precise spatial and temporal pattern of expression of Tbx3, we completely reconstructed its patterns from serial sections. The reconstructions allow the generation of imaginary sections in every plane (Fig. 4A and B). Because the myocardium, lumen components, cushions, and the Tbx3-expressing myocardial and cushion components were separately defined, they can be visualized individually or in every possible combination, and analyzed morphometrically (Fig. 4C and D). Reconstructions were made of chamber-forming hearts (E9.5 and E10.5), septating hearts (E12.5 and E14.5), and the fully septated heart (E17.5). Fig. 5A–E shows proportionally scaled lumen casts and myocardial expression domains of Tbx3. It became apparent that the Tbx3 expression domain is a continuous structure of evolving complexity during development. The volume of the myocardium and the Tbx3-expressing component of the myocardium were measured (Fig. 5F). The volume of the myocardium increased approximately 75-fold between E9.5 and E17.5. The volume of the Tbx3-expressing domain at E9.5 was 10% of the total myocardial volume, and increased 15-fold between E9.5 and E17.5 to be 2% of the total myocardial volume at E17.5.
Overview of reconstructions of mouse hearts. (A)–(E) show proportionally sized ventral views of reconstructions of an E9.5, E10.5, E12.5, E14.5, and E17.5 mouse heart. The myocardium has been removed, revealing the lumen. The Tbx3-expressing myocardium is shown in red. Color usage is the same for all stages and all subsequent figures. Panel F shows quantification of the volume of the total myocardium and the Tbx3-expressing domain in cubic millimeters. The ratio of the myocardial Tbx3-expressing volume and the total myocardial volume is given as a percentage. The data are also shown in a logarithmic graph. Bar=0.5 mm. CCV, common caval vein; RAVJ, right atrioventricular junction; PV, pulmonary vein. For other abbreviations, see legends to Fig. 1.
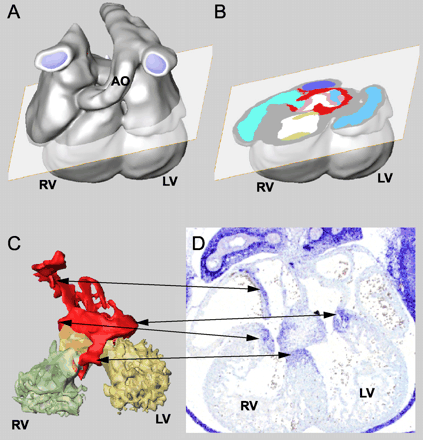
Method of three-dimensional reconstruction of the Tbx3 expression pattern in the heart. (A) Three-dimensional reconstruction of serial sections of an E12.5 mouse heart, the plane of an imaginary section is shown. (B) Imaginary section showing myocardial (red) and cushion/valve (pink) Tbx3 expression in atrioventricular canal region. (C) Each component has been defined and colored separately and can be visualized or removed. Arrows depict Tbx3 expressing parts are also visible in the section of an E12.5 heart in panel D, which has not been used for the reconstruction. LV, left ventricle; RV, right ventricle; AO, aorta.
We next analyzed the reconstructions in more detail. At E9.5, the myocardial Tbx3-expressing domain constitutes a continuous ring around the AVC (Fig. 6A). The weak Tbx3 expression at the IV ring (see Fig. 1E–G) could not be detected at this stage in section ISH. At E10.5, the domain of Tbx3 expression expands toward the dorsal and ventral sides of the interventricular septum and, at E12.5, the entire top of the septum expresses Tbx3(Fig. 6B–F). The dorsal aspect of this region will give rise to the AVB. Between E14.5 and E17.5, the ventral connection between the AVC and AVB is broken (Fig. 6G–I). As a result, the only connection that remains is at the dorsal side between the AV nodal region and the AVB. Also the left atrioventricular domain of Tbx3 expression disappears within these stages, whereas expression in the RAVRB is maintained (Fig. 6E and F).
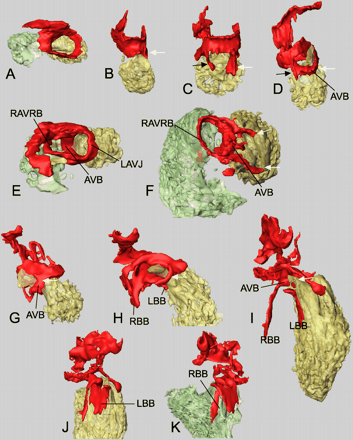
Progressive development of the Tbx3 expression domain in the atrioventricular canal, atrioventricular bundle, and bundle branches (see reconstructions of Fig. 6 for color usage and orientations). (A and B) E9.5, (C) E10.5, (D and E) E12.5, (F) E17.5, (G) E12.5, (H) E14.5, (I–K) E17.5. Arrows in (B)–(D) and (G)–(I) indicate the ventral (white) and dorsal (black) extensions of Tbx3 expression in the interventricular septum. For abbreviations, see legends to Figs. 1 and 5.
Starting at E12.5, the Tbx3 domain of expression extends from the top of the septum along the flanks towards the apex (Fig. 6G–I). This expansion correlates with the formation of the BB. At E17.5, the expression domain reaches approximately halfway to the septum, indicating that only the proximal portion of the BB expresses Tbx3 at very low levels. The Tbx3 expression domain at the left side of the septum is much broader than at the right side of the septum (Fig. 6J and K). This correlates with anatomical data—the left BB forming a sheet and the right bundle forming a real branch [25].
At the dorsal side of the atrium, the myocardial Tbx3 expression domain extends from the AVC towards the sinus venosus (Fig. 7). At E9.5, the extension reaches up to the left and right sinus horns (Fig. 7A and B). Because of the remodeling of the venous pole, the right sinus horn becomes the only connection to the right atrium, whereas the left sinus horn drains into the right sinus horn. This developmentally increasingly asymmetric configuration is reflected by an increasingly asymmetric extension of the Tbx3 expression domain towards the right side of the heart (compare E9.5 and E10.5; Fig. 7A and C). Venous valves flank the entrance of the right sinus horn to the right atrium. At E12.5, the dorsal portion of the valves expresses Tbx3(Fig. 7E). The Tbx3-expressing domain reaches all the way up to the superior caval vein (Fig. 7E and F). This region includes the SAN that will become recognizable morphologically at later stages. The dorsal aspect of the left venous valve and the primary atrial septum also express Tbx3 at this stage. This situation does not significantly change up to E17.5 (Fig. 7G and H). At this stage, the right common cardinal vein has become incorporated into the right atrium, forming the sinus venarum by which the superior and inferior caval veins drain separately to the right atrial chamber. Myocardial Tbx3 expression is contiguous from the sinus region via the terminal crest to the region of the AVC where the AVN will develop. Although at the left side of the sinoatrial connection (left venous valve and the basis of the atrial septum) some Tbx3 expression is observed, no contiguous tracts from the sinoatrial region to the atrioventricular region were observed at this side.
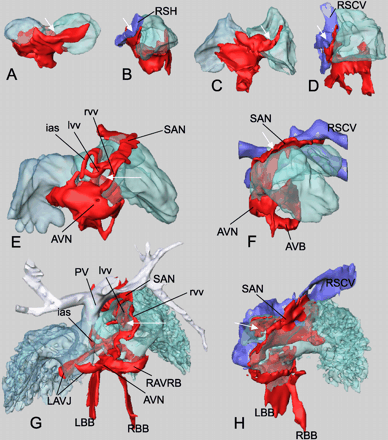
Progressive development of the Tbx3 expression domain in the dorsal wall of the atrium, venous valves, septum, and caval veins. Dorsal and right lateral views. (A and B) E9.5, (C and D) E10.5, (E and F) E12.5, (G and H) E17.5. White arrows indicate opening from caval vein to the right atrium. l/rvv, left/right venous valve. For other abbreviations, see legends to Figs. 1 and 5.
4. Discussion
The key new finding of this study is that Tbx3, encoding a transcriptional repressor, is a novel and unique marker for the entire CCS (SAN, AVN, AVB, and proximal BB) and, as such, facilitates our understanding of the mechanisms underlying its development. Tbx3 is expressed in the area that does not form chamber myocardium, in a pattern mutually exclusive to that of chamber-specific genes. Furthermore, Tbx3 is able to repress chamber-specific promoters. Therefore, we propose that one function of Tbx3 is the continuous suppression of the chamber phenotype in specific myocardial areas, allowing these areas to form the CCS.
4.1. The Tbx3 transcriptional domain: developmental implications
From E8.5 onwards, mouse Tbx3 is expressed in the sinoatrial region, AVC, and interventricular ring. This early expression pattern provides molecular evidence that the CCS precursors exist before its components become histologically recognizable [26,27]. This is in agreement with electrophysiological observations of pacemaker cells in the early sinus venosus [28] and the presence of atrioventricular delay before the development of a morphologically identifiable AVN [29,30].
Whether the conduction network is established through outgrowth of a prespecified pool of primary cardiomyogenic progenitors [1,3,9] or through continuous recruitment from neighboring chamber myocardial cells until late in development [2] is subject of an ongoing debate. The results presented here are consistent with the first notion.
Our observations indicate that the precursor pool proliferates slowly because the volume of the Tbx3-expressing pool increases much less than that of the chambers (see Fig. 5). In agreement, the IFT and AVC precursor region and derived CCS components display low proliferative activity [2,31].
4.2. Tbx2 and Tbx3 repress chamber differentiation
The Tbx3-expressing domain does not express chamber-type myocardial genes, suggesting a role for Tbx3 in repressing the chamber–myocardial phenotype. Our data show that similar to Tbx2, Tbx3 represses chamber-specific promoters. Tbx3 represses a Tbx5/Nkx2.5-activated promoter and forms a complex with Nkx2.5 on the Nppa promoter. Furthermore, Tbx3 was found to block myogenic differentiation of C2C12 myoblasts and, as a result, myosin gene expression [32]. This is consistent with the observation that compared to the working myocardium of the chambers, the Tbx3-expressing nodes, AVB and BBs, express lower levels of myosin genes and have a less well-developed contractile apparatus, reminiscent of the primary myocardium of the embryonic heart [6]. We propose that like Tbx2, Tbx3 represses chamber-specific gene expression and chamber–myocardial differentiation. At late fetal stages, Cx40 expression is initiated in the proximal BB, and in the AVB shortly before birth. Tbx3 is expressed at relatively low levels in these structures (not shown) and may not be able to compete effectively with Tbx5. However, further studies are required to explain the selective escape of Cx40 from repression in the AVB and proximal BB late in development.
Humans heterozygous for TBX3 mutations or mice deficient for Tbx3 have no reported cardiac phenotype [23,33]. The overlapping expression patterns and functional redundancy between Tbx2 and Tbx3 may be the basis for the lack of an overt cardiac phenotype in Tbx3 mutants.
4.3. The topography of the expression of Tbx3 delineates the central conduction system
Reconstructions revealed that the Tbx3 gene is expressed in all components of the CCS. However, the expression pattern also includes the internodal regions, and the left, ventral and right sides of the AVC, which are usually not included in the CCS.
4.3.1. Internodal tracts
In agreement with the description of the sinoatrial ring in developing human embryos by Wenink [34], the strongest expression of Tbx3 is observed at the right side of the sinus muscle. This region corresponds with the terminal crest, which forms the boundary between the right embryonic atrium (future atrial appendage) and the sinus venarum (=dorsal right atrial wall formed by incorporation of the sinus venosus) and extends from the Tbx3-positive SAN to the Tbx3-positive AVN/atrioventricular canal. The existence of internodal tracts has been subject of vigorous debate [35–37]. Tracts defined as insulated internodal pathways have never been described; also the electrophysiological significance has been much debated [35]. We now describe a prominent Tbx3-expressing region at the position of the terminal crest that interconnects the nodes, classifying this myocardium as ‘nonchamber.’ We consider this region of slow conduction (low levels of connexin expression) as ‘vestigial’ without much function in the normal mature heart.
4.3.2. Atrioventricular canal
Tbx3 unambiguously identifies the atrioventricular region as a distinct component of the atrial chambers that is not composed of working myocardium as evident from the absence of expression of chamber-type myocardial markers. Moreover, the absence of expression of Cx40 and Cx43, encoding the main cardiac gap junctions required for intercellular conduction, is compatible with low conduction velocities in this area, typical for nodal-like tissue. In the formed heart, this region of smooth-walled myocardium constitutes the lower rim of the atria. Although it is well accepted that in the embryonic heart the dorsal aspect of this region will give rise to the atrioventricular nodal tissues [9,27,37,38], the functional significance of the other parts of this region has remained indeterminate. However, McGuire et al. [39] have described this region as having “nodal” characteristics. Ectopic nodes have been found in the entire lower rim of both the right and the left atria in congenitally malformed hearts [37]. In the normal mature heart, we consider this region to have no specific function because AV insulation has been achieved by the formation of fibrous tissue.
Recent molecular studies have identified both the internodal region and the atrioventricular canal as being intimately associated with the CCS, supporting the morphological and physiological data and our expression data on Tbx3. Firstly, Rentschler et al. [3,40] have described a molecular marker, the reporter gene engrailed LacZ in mouse, that appears to identify not only all components of the classical cardiac conduction system but also the internodal zone and the entire atrioventricular junctional area. This indicates that all these ‘conduction system’ components share a common transcriptional control mechanism. Characterization of the site of integration of this reporter gene will eventually provide insight into the molecular mechanism underlying the selective transgene transcription pattern. Secondly, expression of Cx45 can be recognized in all components of the conduction system including the terminal crest connecting the SAN and AVN, the atrioventricular region, and the OFT [41–43]. Therefore, similar to Tbx3, Cx45 identifies the myocardium of the CCS and the associated areas discussed above.
Acknowledgements
We thank Drs V. Papaioannou, M. van Lohuizen, M. Nemer, B. Bruneau, R. Harvey, C. Basson and J. Izpisua Belmonte for probes and plasmids, Dr. J. Ruijter for help with the reconstructions, D. Clout, C. de Gier-de Vries and Mara Pietrobon for technical assistance and Dr. P. Barnett for critically reading the manuscript. This study was supported by the Netherlands Heart Foundation M96.002.
References
Author notes
These authors contributed equally to this work.
Time for primary review 21 days