-
PDF
- Split View
-
Views
-
Cite
Cite
Martin F. Reiner, Alexander Akhmedov, Simona Stivala, Stephan Keller, Daniel S. Gaul, Nicole R. Bonetti, Gianluigi Savarese, Martina Glanzmann, Cuicui Zhu, Wolfram Ruf, Zhihong Yang, Christian M. Matter, Thomas F. Lüscher, Giovanni G. Camici, Juerg H. Beer, Ticagrelor, but not clopidogrel, reduces arterial thrombosis via endothelial tissue factor suppression, Cardiovascular Research, Volume 113, Issue 1, January 2017, Pages 61–69, https://doi.org/10.1093/cvr/cvw233
Close - Share Icon Share
The P2Y12 antagonist ticagrelor reduces mortality in patients with acute coronary syndrome (ACS), compared with clopidogrel, and the mechanisms underlying this effect are not clearly understood. Arterial thrombosis is the key event in ACS; however, direct vascular effects of either ticagrelor or clopidogrel with focus on arterial thrombosis and its key trigger tissue factor have not been previously investigated.
Human aortic endothelial cells were treated with ticagrelor or clopidogrel active metabolite (CAM) and stimulated with tumour necrosis factor-alpha (TNF-α); effects on procoagulant tissue factor (TF) expression and activity, its counter-player TF pathway inhibitor (TFPI) and the underlying mechanisms were determined. Further, arterial thrombosis by photochemical injury of the common carotid artery, and TF expression in the murine endothelium were examined in C57BL/6 mice treated with ticagrelor or clopidogrel. Ticagrelor, but not CAM, reduced TNF-α-induced TF expression via proteasomal degradation and TF activity, independently of the P2Y12 receptor and the equilibrative nucleoside transporter 1 (ENT1), an additional target of ticagrelor. In C57BL/6 mice, ticagrelor prolonged time to arterial occlusion, compared with clopidogrel, despite comparable antiplatelet effects. In line with our in vitro results, ticagrelor, but not clopidogrel, reduced TF expression in the endothelium of murine arteries.
Ticagrelor, unlike clopidogrel, exhibits endothelial-specific antithrombotic properties and blunts arterial thrombus formation. The additional antithrombotic properties displayed by ticagrelor may explain its greater efficacy in reducing thrombotic events in clinical trials. These findings may provide the basis for new indications for ticagrelor.
1. Introduction
Cardiovascular disease is the leading cause of death worldwide.1 Platelets play a crucial role in arterial thrombus formation, the key event in cardiovascular complications, such as myocardial infarction and stroke. Consequently, platelet antagonists targeting the adenosine diphosphate (ADP) receptor P2Y12 (i.e. clopidogrel, prasugrel, or ticagrelor) in combination with acetylsalicylic acid is the treatment of choice for patients suffering from acute coronary syndrome (ACS) and for secondary prevention after stent implantation or coronary artery bypass grafting.2 In clinical trials, ticagrelor and prasugrel proved to be superior over clopidogrel;3,4 in particular, ticagrelor reduced the composite end point mortality due to vascular causes, myocardial infarction, or stroke in addition to overall mortality in patients with ACS, compared with clopidogrel.3 The cyclopentyl-triazolo-pyrimidine ticagrelor displays a different pharmacokinetic and pharmacodynamic profile, compared with the thienopyridines clopidogrel and prasugrel. Specifically, ticagrelor is direct acting and reversibly binding the P2Y12 receptor, whereas the thienopyridines are pro-drugs requiring hepatic metabolism to generate an irreversibly binding active metabolite. Higher levels of platelet inhibition complemented by an additional mechanism of action, the inhibition of cellular adenosine uptake via the equilibrative nucleoside transporter 1 (ENT1)5–8 leading to increased adenosine plasma levels,9 may in part account for the superior effects of ticagrelor.
A growing body of evidence suggests off-target effects of P2Y12 antagonists; however, effects of ticagrelor and clopidogrel on the endothelium, their underlying molecular mechanisms and potential effects on arterial thrombosis remain elusive.
Tissue factor (TF) is the key trigger of the extrinsic coagulation cascade10 and plays a major role in the development of arterial thrombotic complications such as myocardial infarction and stroke.11 Correspondingly, anti-TF antibody treatment blocks arterial thrombus formation.12 Increased levels of TF have been observed in patients with classical cardiovascular risk factors, such as hypertension,13 diabetes,14 and dyslipidaemia,15 as well as in ACS.16 TF is expressed in various cell types including endothelial cells and different cytokines such as tumour necrosis factor-alpha (TNF-α) are known to induce TF expression and activity.17 Correspondingly, elevated levels of TNF-α have also been reported in patients with ACS18 and atrial fibrillation19 and are further associated with cardiovascular disease progression and severity.20
In the present study, we investigate pleiotropic effects of ticagrelor and clopidogrel on TNF-α-stimulated primary human aortic endothelial cells (HAECs), assess the underlying mechanisms and their potential relevance in a mouse model of photochemical injury-induced arterial thrombosis.
2. Methods
2.1 Drugs
Clopidogrel active metabolite (CAM) and ticagrelor were provided by Sanofi-Aventis, Germany and AstraZeneca, Mölndal, Sweden, respectively. Clopidogrel was purchased from Santa Cruz Biotechnology.
2.2 Animals and treatment
Twelve-week-old bodyweight-matched male C57BL/6 mice were treated with clopidogrel (48 mg/kg/day or 32 mg/kg/day) or ticagrelor (120 mg/kg/day or 80 mg/kg/day) given in the chow (clopidogrel, 0.06% w/w or 0.04 w/w; ticagrelor, 0.15% w/w or 0.1% w/w), for 2 weeks to evaluate dosages that provide full and comparable platelet inhibition in mice. Dosages of clopidogrel and ticagrelor differed 2.5-fold corresponding to dosages used in humans2 and dosages known to provide comparable platelet inhibitory effects in rodents.21 Experimental procedures involving mice were reviewed and approved by the institutional animal care committee (licence number TVA 165/2013; Kommission für Tierversuche des Kantons Zürich, Zurich, Switzerland).
2.3 Plasma concentrations of ticagrelor
In addition to pharmacodynamic evaluation of P2Y12 receptor antagonist treatment, ticagrelor plasma concentrations were determined by protein precipitation and liquid chromatography mass spectrometry as described previously.22
2.4 Whole blood aggregometry
Sodium-citrate (3.8%) anticoagulated blood was collected by cardiac puncture using a 24-gauge needle after euthanasia of mice by isoflurane. Finally, whole blood (diluted 1–3 in 0.9% saline) aggregometry was performed in response to ADP (10 µM; 384, chrono-log), thrombin (1 U/mL; T7009, Sigma Aldrich) and collagen (10 μg/mL; Kollagenreagens Horm, Takeda) by impedance aggregometer (Chrono-Log).
2.5 Arterial thrombosis
After 2 weeks of treatment with ticagrelor, clopidogrel, or control chow, C57BL6 mice were exposed to photochemical injury of the common carotid artery (CCA) as previously described.23 Briefly, mice were anaesthetized using pentobarbital (87 mg/kg body weight); after midline neck incision, the right CCA was exposed under an operating microscope. To induce photochemical injury of the endothelium, bengal rose (50 mg/kg body weight) was injected into the tail vein and the CCA was exposed to a laser light beam (1.5 mW, 540 nm, Mellesgriot Inc.) up to 120 min. Blood flow and heart rate were monitored (Doppler flow probe carotid artery Transonic Systems Inc., 0.5VB) until occlusion occurred or for a maximum of 120 min, in case arterial thrombosis was not detected.
2.6 Plasma thrombin generation
Plasma thrombin generation was assessed by calibrated automated thrombogram as previously described.24 Sodium-citrate (3.8%) anticoagulated murine whole blood was drawn by cardiac puncture and centrifuged (4000g, 10 min, 4 °C) to receive platelet-poor plasma, which was then mixed with either PPP-Reagent (Thrombinoscope BV) containing TF (f.c. 6 pM) and phospholipids (f.c. 4.8 μM), or thrombin calibrator (Thrombinoscope BV). Next, fluorogenic thrombin substrate (Thrombinoscope BV), Fluo-Buffer (Hepes buffer, pH 7.35, 20 mM Hepes, with BSA 60 mg/mL, Thrombinoscope BV), and CaCl2 (246 mM) were added. Thrombin generation was measured over time by Fluoroskan® Ascent reader (Thermo Labsystems) and thrombin generation curve was calculated by Thrombinoscope software (Thrombinoscope BV) to finally receive endogenous thrombin potential (ETP) (nmol thrombin x min).
2.7 Endothelial tissue factor expression
CCAs of mice were embedded in Optimal Cutting Temperature medium, snap frozen and cryosectioned. Sections were incubated with the primary antibodies rat anti-murine CD31 (BD 553370, 1:2500), used as an endothelial cell marker, and rabbit anti-TF (R8084,25 1:200) for 45 min at room temperature. After washing, the secondary antibodies donkey anti-rat Cy3 (Jackson 712-166-153, 1:250) and donkey anti-rabbit Alexa488 (Jackson 711-545-15, 1:250) were applied for 45 min at room temperature. DNA was counterstained using UltraCruz Hard-set Mounting medium containing 4',6-diamidino-2-phenylindole (Santa Cruz, sc-359850). Images were acquired using a Zeiss Axiovert inverted widefield fluorescence microscope with Axiovision software. Composite images were generated using Photoshop (CC2015, Adobe) and endothelial TF mean fluorescent intensity was quantified and normalized to endothelial cell area.
2.8 Isolation of human platelets
Human platelets were used as positive control of P2Y12 receptor mRNA and protein expression. Venous blood was drawn from a healthy volunteer not receiving any medication for 10 days and collected in sodium-citrate (3.8%) tubes (Becton Dickinson). Blood was centrifuged twice at 100g for 10 min at room temperature to obtain platelet-rich plasma, which was centrifuged at 400g for 15 min at room temperature. Finally, the platelet pellet was washed with 1 mL phosphate-buffered saline and after a final centrifugation step (400g, 10 min, room temperature) exposed to either protein lysis buffer or TRIzol for Western blot or real-time polymerase chain reaction (RT-PCR), respectively.
2.9 Cell culture experiments
HAECs (Lonza) were used for experiments between Passages 5 and 8, derived from four individual batches. Endothelial cells were cultured in endothelial growth basal medium-2, supplemented with endothelial growth basal medium-2 bullet kit (Lonza) and 10% foetal bovine serum. After 24 h of growth, cells underwent starvation for 24 h using endothelial basal medium (Lonza) supplied with 0.5% foetal bovine serum. Cells were treated with concentration ranges of ticagrelor (10−7, 10−6, 10−5 M)26 or CAM (1.5 × 10 − 8, 1.5 × 10 − 7, 1.5 × 10 − 6 M), which are in line with plasma concentrations found in humans,26 and stimulated with TNF-α (10 ng/mL) for various time points. Drugs were dissolved in dimethyl sulfoxide (f.c. 0.1%). Correspondingly, unstimulated cells and TNF-α-stimulated cells were treated with dimethyl sulfoxide (f.c. 0.1%) to exclude vehicle-dependent effects.
2.10 Western blotting
Protein expression was determined by Western blot analysis. Endothelial cells and human platelets were incubated with lysis buffer (NaCl 150 mM, EDTA 1 mM, NaF 1 mM, DTT 1 mM, aprotinin 10 mg/mL, leupeptin 10 mg/mL, Na3VO4 0.1 mM, PMSF 1 mM, and NP-40 0.5%); protein concentration was determined, according to the recommendations of the manufacturer (Bio-Rad); 20–30 µg of protein lysates were separated on an 8% or 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis before being transferred to a polyvinylidene fluoride membrane by semi-dry transfer. Membranes were cut, according to the size of proteins of interest and incubated with primary antibodies overnight at 4 °C on a shaker. Secondary antibodies were applied for 1 h at room temperature. Densitometric analyses were performed and protein expression was normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Antibodies against TF (ADG4507 and 4503; 1:2000) and tissue factor pathway inhibitor (TFPI) (ADG72; 1:8000) were purchased from American Diagnostica; anti-P2Y12 (ab86195; 1:2000) antibody from Abcam; anti-GAPDH antibody (MAB374; 1:40 000) from Merck Millipore. Secondary anti-mouse (1031-05) and anti-rabbit (4050-05) antibodies were obtained from SouthernBiotech; recombinant human TNF-α (210-TA) was purchased from R&D Systems; actinomycin D (A1410), MG132 (C2211), rapamycin (R0395), wortmannin (W1628), SB203580 (S8307), SP600125 (S5567), dipyridamole (D9766), adenosine receptor antagonist against A1 (8-cyclopentyl-1,3-dipropylxanthine), A2a (SCH 442416), A2b (MRS 1754), and A3 (VUF 5574) as well as adenosine (A4036) from Sigma Aldrich; cycloheximide (239765) from Merck Millipore; PD 98059 (9900) from Cell Signaling Technology.
2.11 Real-time PCR
Total RNA was extracted from HAECs or human platelets using TRI reagent (Sigma Aldrich), according to the recommendations of the manufacturer. Conversion of the total cellular RNA to cDNA was performed with Moloney murine leukaemia virus reverse transcriptase and random hexamers (GE Healthcare) in a final volume of 35 μL, using 2 μg of total RNA, according to recommendations of the manufacturer. RT-PCR was performed in a QuantStudio 7 Flex RT-PCR cycler (Applied Biosystems), according to the instructions of the manufacturer. All RT-PCR experiments were performed using the SYBR Select Master Mix provided by Applied Biosystems (Life Technologies). Each reaction (20 μL) contained 2 μL cDNA, 400 fmol of each primer and 10 μL of Master Mix. The following primers were used: P2Y12 (1), P2Y12 (2), TF, human L28, and human β-actin (ACTB). The amplification program consisted of 1 cycle at 95°C for 10 min, followed by 40 cycles with a denaturing phase at 95°C for 15 s, an annealing/elongation phase at 60°C for 1 min. A melting curve analysis was performed after amplification to verify the accuracy of the amplicon. For verification of the correct amplification, PCR products were analysed on an ethidium bromide stained 1.5% agarose gel. Cycle threshold (CT) values for each gene were obtained for each sample and differences in CT values between a test gene and endogenous controls (ΔCT) were calculated and used for statistical analyses.
For quantitative RT-PCR, the following primers were used; for human P2Y12: sense primer (1) 5′-CTTTCTCATGTCCAGGGT-3′, antisense primer (1) 5′-GTTGCCAAACCTCTTTGT-3′; sense primer (2) 5′-TTTCTCATGTCCAGGGTC-3′, antisense primer (2) 5′-CTGCAGAGTGGCATCTGG-3; for human TF: sense primer: 5′-CCAAACCCGTCAATCAAGTC-3′, antisense primer: 5′-TGCCAAGTACGTCTGCTTCA-3′; for human L28: sense primer: 5′-GCATCTGCAATGGATGGT-3′, antisense primer: 5′-TGTTCTTGCGGATCATGTGT-3′; for ACTB: sense primer: 5′-GCACAGAGCCTCGCCTT-3′, antisense primer: 5′-GTTGTCGACGACGAGCG-3′. All primers were ordered from Microsynth.
2.12 Tissue factor activity assay
TF activity was determined as previously described23 in cell lysates of HAECs and murine plasma by ELISA, according to the recommendations of the manufacturer (Sekisui Diagnostics, ACTICHROME® TF, 846). Endothelial cells were lysed (50 mmol/L Tris–HCl, 100 mmol/L NaCl, 0.1% Triton X-100, pH 7.4), diluted 1:15 in assay buffer, and mixed with human factor VIIa and X, which leads to the conversion of factor X to Xa; factor Xa subsequently cleaves the chromogenic substrate SPECTROZYME® FXa. Finally, absorbance was measured at 405 nm and after background subtraction, optical density was normalized to protein concentration as determined by Nanodrop 2000 Spectrophotometer (Thermo Scientific). Sodium-citrate (3.8%) anticoagulated plasma was mixed with factor VIIa and X and optical density of cleaved SPECTROZYME® FXa was determined at 490 nm and subtracted from absorbance at 405 nm. Finally, plasma TF (pM) was calculated, according to a standard curve.
2.13 Tissue factor mRNA stability
Stability of TF mRNA was investigated as previously described.27 Endothelial cells were stimulated with TNF-α for 1 h to induce TF gene expression and transcription was subsequently terminated using actinomycin D (10 µg/mL). Next, cells were incubated with ticagrelor (10−5 M) for 1, 2, or 3 h before cells were lysed with TRIzol. Messenger RNA was isolated and RT-PCR was performed. Values were plotted as per cent of time 0 against time (min) and half-life was calculated by non-linear regression using Prism 6 (GraphPad software).
2.14 Statistical analysis
Data are expressed as mean ± SEM. Statistical analysis was performed using one-way ANOVA with Tukey post hoc test or unpaired two-tailed Student’s t-test as appropriate. A probability value below or equal to 0.05 was considered as statistically significant and calculated by Prism 6 (GraphPad software).
3. Results
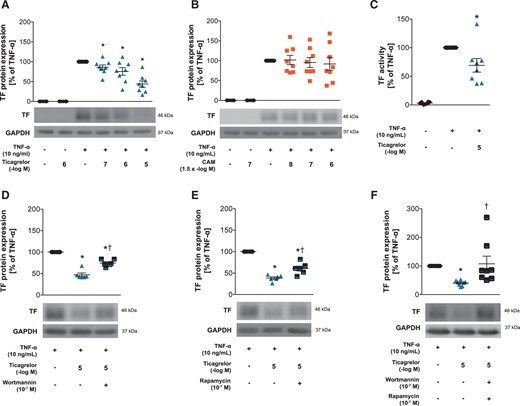
3.1 Ticagrelor, but not CAM, reduces TF expression and activity
Effects of ticagrelor and CAM on TF expression and activity in HAECs. TF protein expression in ticagrelor-pretreated HAECs (n = 8) (A), or CAM-pretreated HAECs (n = 8) (B) 1 h before stimulation with TNF-α for 5 h. (C) TF activity in ticagrelor-pretreated HAECs 1 h before TNF-α stimulation for 5 h (n = 8). TF protein expression in HAECs pretreated with the PI3 kinase inhibitor wortmannin (n = 6) (D), the p70s6 kinase inhibitor rapamycin (n = 6) (E) or both (n = 8) (F) 1 h prior to ticagrelor treatment for 1 h and subsequent TNF-α stimulation for 5 h. *P <0.05 vs. TNF-α treatment; †P <0.05 vs. TNF-α + ticagrelor treatment. CAM, clopidogrel active metabolite; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; HAECs, human aortic endothelial cells; TF, tissue factor; TNF-α, tumour necrosis factor-alpha.
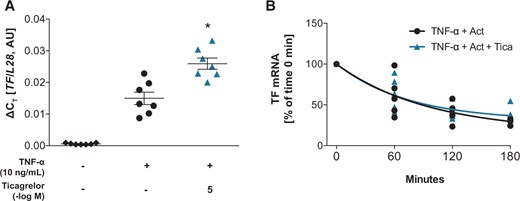
3.2 Ticagrelor decreases endothelial TF by proteasomal degradation
Ticagrelor augmented TF transcription but did not alter mRNA half-life in HAECs. (A) TF mRNA levels in HAECS after preincubation with ticagrelor 1 h before TNF-α-stimulation for 3 h (n = 7). (B) TF mRNA stability after treatment with TNF-α (10 ng/mL) for 1 h and subsequent simultaneous incubation with actinomycin D (10 µg/mL) and ticagrelor (n = 4–6) for time points between 0 and 180 min. *P <0.05 vs. TNF-α treatment. Act, actinomycin D; AU, arbitrary unit; HAECs, human aortic endothelial cells; TF, tissue factor; Tica, ticagrelor; TNF-α, tumour necrosis factor-alpha.
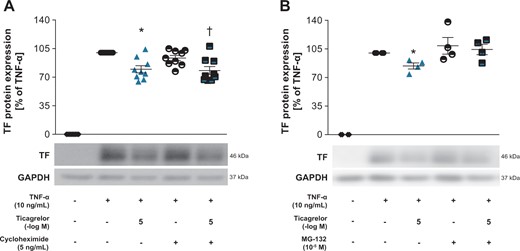
Ticagrelor reduces TF by proteasomal degradation in HAECs. (A) TF expression in HAECs stimulated with TNF-α for 3 h followed by simultaneous treatment with the protein translation inhibitor cycloheximide and ticagrelor for additional 2 h (n = 9). (B) TF protein expression in HAECs stimulated with TNF-α-for 3 h and treated with the proteasome inhibitor MG-132 and ticagrelor simultaneously for additional 2 h (n = 4). *P <0.05 vs. TNF-α treatment. †P <0.05 vs. TNF-α + cycloheximide treatment. GAPDH, glyceraldehyde 3-phosphate dehydrogenase; TF, tissue factor; TNF-α, tumour necrosis factor-alpha.
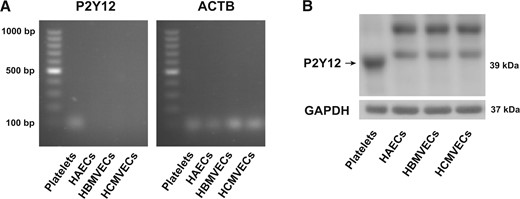
3.3 Effects of ticagrelor are mediated independently of P2Y12 or ENT1
Presence of P2Y12 receptor in human platelets but not in endothelial cells. (A) P2Y12 RNA in human platelets compared with HAECs, HBMVECs and HCMVECs (n = 3). (B) Protein expression of the P2Y12 receptor in human platelets, compared with HAECs, HBMVECs, or HCMVECs (n = 3). ACTB, human β-actin; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; HAECs, human aortic endothelial cells; HBMVECs, human brain microvascular endothelial cells; HCMVECs, human cardiac microvascular endothelial cells.
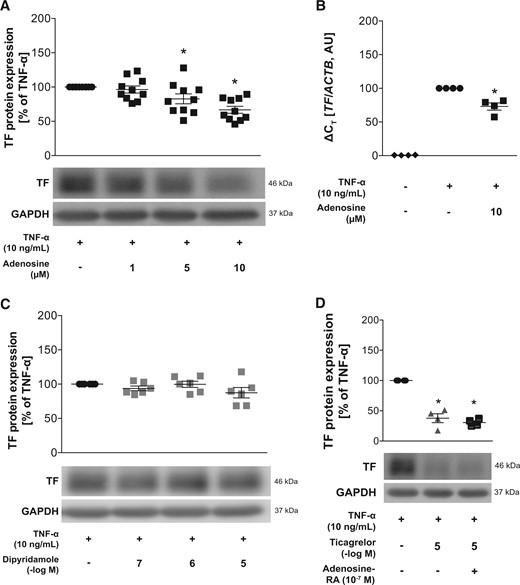
Effects of adenosine, dipyridamole and adenosine receptor antagonists on TF expression. (A) Effects of adenosine on TF protein (n = 10) and (B) RNA expression (n = 4) after pretreatment for 1 h and subsequent stimulation with TNF-α for 5 and 3 h, respectively. (C) Effects of the ENT1 inhibitor dipyridamole on TF expression in HAECs after pretreatment for 1 h and subsequent stimulation with TNF-α for 5 h (n = 6). (D) Pretreatment of HAECs with adenosine receptor antagonists for 1 h prior to ticagrelor treatment for 1 additional hour and subsequent TNF-α stimulation for 5 h (n = 4). *P <0.05 vs. TNF-α treatment. ACTB, human β-actin; ENT1, equilibrative nucleoside transporter 1; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; HAECs, human aortic endothelial cells; RA, receptor antagonists; TF, tissue factor; TNF-α, tumour necrosis factor-alpha.
3.4 Ticagrelor decreases arterial thrombosis and endothelial TF expression in mice
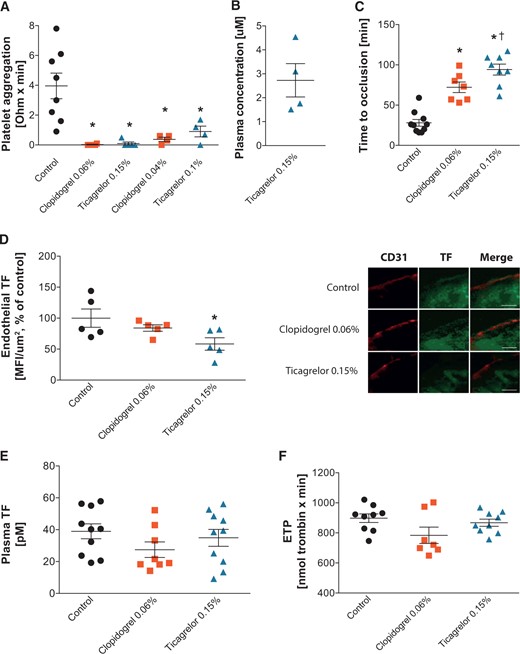
Platelet aggregation, endothelial TF expression, time to arterial occlusion, and thrombin potential. (A) Platelet aggregation in response to ADP (10 μM) in mice treated with control chow, clopidogrel, or ticagrelor for 2 weeks. (B) Plasma concentration of ticagrelor in mice treated with ticagrelor 0.15% added to chow. (C) Time to arterial thrombosis in mice treated with control chow (n = 10), clopidogrel (n = 7), or ticagrelor (n = 8). (D) Endothelial TF expression in CCAs of mice treated with control chow, clopidogrel, or ticagrelor (n = 5) and representative transverse sections of CCA stained for endothelium (CD31, red, scale bar 5 µm) and TF (green, scale bar 5 µm) (E). Plasma TF activity and (F) plasma thrombin potential clopidogrel-, ticagrelor-, and control chow-treated animals at baseline. *P <0.05 vs. control; †P <0.05 vs. clopidogrel. ADP, adenosine diphosphate; CCA, common carotid artery; ETP, endogenous thrombin potential; MFI, mean fluorescence intensity; TF, tissue factor.
4. Discussion
Different P2Y12 receptor antagonists have been shown to differentially affect cardiovascular outcome in several clinical trials.3,4 Particularly, the Study of Platelet Inhibition and Patient Outcomes (PLATO) trial showed superior effects of ticagrelor, compared with clopidogrel, in reducing mortality in patients with ACS.3 Stronger antiplatelet effects and reversible binding, which further allows inhibition of newly formed platelets and micro particles, may partly account for the greater efficacy of ticagrelor. Nonetheless, increased platelet inhibition achieved by prasugrel, compared with clopidogrel, did not translate into a similar reduction of overall mortality suggesting the possible involvement of platelet-independent effects.4 In line with this, we hypothesized that ticagrelor may prevent endothelial activation with a specific effect on arterial thrombus formation and its key player TF; this indeed could provide a plausible explanation for the reduction in thrombotic events observed in PLATO.
Indeed, in TNF-α-stimulated HAECs, we found that ticagrelor, but not CAM, reduced TF expression via proteasomal degradation and TF activity without affecting TFPI. Interestingly, we found that these effects were mediated independently of both the P2Y12 receptor and the adenosine transporter ENT1. To test the physiological relevance of our in vitro findings, we induced arterial thrombosis in C57BL/6 mice treated with ticagrelor or clopidogrel. Dosages were chosen to mimic human plasma concentrations21 and to induce comparable platelet inhibition in both groups and, thus, to exclude platelet-dependent effects. Notably, we found that time to arterial occlusion was significantly delayed in ticagrelor-treated mice, compared with clopidogrel-treated rodents. In line with our in vitro findings ticagrelor, but not clopidogrel, reduced protein expression of endothelial TF in murine arteries. Plasma coagulation parameters including thrombin generation and tissue factor activity on the other hand remained unchanged, which further supports the hypothesis of local vascular antithrombotic mechanisms.
The herein reported findings support our hypothesis of pleiotropic effects of ticagrelor on endothelial cells and expand previous studies describing P2Y12-independent effects of ticagrelor.7,8 The inhibitory effect on TF displayed by ticagrelor may be particularly favourable in patients at risk of cardiovascular complications, as both activation of platelets and activation of the coagulation cascade represent key mechanisms in the initiation and propagation of arterial thrombus formation as it occurs in ACS. This notion may contribute to the observed decrease in arterial thrombotic events.3
Unlike previous observations,8,21 the herein reported mechanisms cannot be explained by increased adenosine levels following ENT1 inhibition. Although adenosine mimics the effect of ticagrelor, we did not observe similar results using the ENT1 inhibitor dipyridamole. Additionally, adenosine receptor antagonists could not reverse ticagrelor’s effect; thus, implying a different mechanism. Correspondingly, patients receiving ticagrelor or adenosine show similar side effects, such as the induction of dyspnea,3 and the recent finding of increased adenosine levels8 through ENT1 inhibition7 provides evidence to link these observations. Nevertheless, potent ENT1 inhibitors such as dipyridamole also increase adenosine levels,8 but do not induce dyspnea.31 Therefore, exclusive ENT1 in addition to P2Y12 inhibition may not explain the entire mode of action of ticagrelor and further research is needed to determine its exact mechanisms.
Because of the chemical similarity of ticagrelor and adenosine, direct interactions with adenosine receptors appear to be likely and have been investigated by others; however, such interactions were considered unlikely to be clinically relevant because of the low affinity of ticagrelor.7 The P2Y12 receptor belongs to the family of P2 purine and pyrimidine G protein-coupled receptors and seven further P2Y receptors have been characterized, some of which are expressed in the endothelium.32 However, whether ticagrelor shows binding affinity to other purine and pyrimidine receptors and whether these receptors are responsible for the observed effects on endothelial cells remains to be determined.
Here we for the first time provide evidence for endothelium-dependent antithrombotic properties, which indicates that ticagrelor can affect the coagulation system in addition to its well-known antiplatelet effects. Dual antiplatelet therapy is the standard of care in patients suffering from ACS2; however, given the high prevalence of this disease,1 there is also a great number of patients with comorbidities requiring anticoagulant treatment, such as deep vein thrombosis, atrial fibrillation, or mechanical heart valves.33 Although anticoagulants in combination with dual antiplatelet therapy lower the risk of thrombotic events, disproportionately increased bleeding rates have been observed.34 While single antiplatelet therapy using clopidogrel in combination with anticoagulants reduced the risk of bleedings,35 such an approach may not be sufficient to sustain the low rate of thrombotic events. Thus, further studies are needed to determine the effectiveness and safety of new oral anticoagulants in combination with more potent platelet antagonists such as ticagrelor.33 Because of its dual antithrombotic effects, ticagrelor may be a more appropriate choice than clopidogrel in patients with ACS with comorbidities requiring additional antithrombotic therapy. Along these lines, ticagrelor showed better efficacy than clopidogrel in the PLATO trial.3 On the other hand, safety in terms of bleeding may be of particular concern in these patients as ticagrelor increased non-procedure-related bleeding rates in the PLATO trial.3
In conclusion, the pleiotropic effects of ticagrelor on the endothelium may in part explain its greater efficacy in reducing thrombotic events in patients with ACS and mortality in clinical trials; its antithrombotic properties may be particularly promising in patients requiring anticoagulant in addition to antiplatelet therapy. Nonetheless, additional antithrombotic properties may increase bleeding risk and special attention may be required in patients at high risk.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was supported by the Swiss National Science Foundation (310030_144152/1 to J.H.B., 310030_147017 to G.G.C., and 310030_166576 to T.F.L.), the Foundation of Cardiovascular Research Zurich, Switzerland, the Foundation Kardio, Baden, Switzerland, and a research grant from Hartmann Müller-Stiftung, Zurich, Switzerland.
Conflict of interest: Ticagrelor and a research grant were provided by AstraZeneca, Mölndal, Sweden and clopidogrel active metabolite by Sanofi-Aventis, Germany GmbH. T.F.L. and J.H.B. have received educational and research grants as well as honoraria from AstraZeneca, Zug, Switzerland.
References
Author notes
Time of primary review: 18 days