-
PDF
- Split View
-
Views
-
Cite
Cite
Benoit Boivin, Catherine Lavoie, George Vaniotis, Alessandra Baragli, Louis-Robert Villeneuve, Nathalie Ethier, Phan Trieu, Bruce G. Allen, Terence E. Hébert, Functional β-adrenergic receptor signalling on nuclear membranes in adult rat and mouse ventricular cardiomyocytes, Cardiovascular Research, Volume 71, Issue 1, July 2006, Pages 69–78, https://doi.org/10.1016/j.cardiores.2006.03.015
Close - Share Icon Share
Abstract
Objective We sought to determine if different β-adrenergic receptor (βAR) subtypes, and their associated signalling machinery, are functionally localized to nuclear membranes.
Methods Employing enriched nuclear preparations, we assayed the specific presence of βAR by measuring 125I-cyanopindolol (CYP) binding, Western blotting, confocal microscopy and functional assays.
Results Western blots of rat heart nuclear fractions and confocal immunofluorescent analysis of adult rat and mouse ventricular cardiomyocytes displayed the presence of β1AR and β3AR but, surprisingly, not the β2AR on nuclear membranes. Nuclear localization of downstream signalling partners Gs, Gi and adenylyl cyclases II and V/VI was also demonstrated. The functional relevance of nuclear βAR was shown by receptor-mediated stimulation of adenylyl cyclase activity by isoproterenol but not the β3AR-selective agonist CL 316243 in enriched nuclear preparations. We also examined the effect of subtype-selective ligands on the initiation of RNA synthesis in isolated nuclei. Both isoproterenol and another β3AR-selective agonist, BRL 37344, increased RNA synthesis which was inhibited by pertussis toxin (PTX). Neither a β1AR-selective agonist, xamoterol, nor a β2AR-selective agonist, procaterol, was able to stimulate transcription. However, both CGP 20712A and ICI 118,551 blocked isoproterenol-mediated effects to varying extents. PTX treatment also revealed that nuclear βAR may be coupled to other signalling pathways in addition to Gi, as stimulation under these conditions reduced initiation of transcription below basal levels.
Conclusion These results highlight differential subcellular localization for βAR subtypes and indicate that βAR may have specific roles in regulating nuclear function in cardiomyocytes.
This article is referred to in the Editorial by J. Neumann (pages 6–7) in this issue.
1 Introduction
An increasing number of GPCRs are targetted to the nuclear membrane, including lysophosphatidic acid receptors [1], metabotropic glutamate receptors (MGluR5, [2]), apelin receptors [3], platelet activating factor (PAF) receptors [4], bradykinin B2 receptors [3], angiotensin 2 type I receptors [3,5–7], prostaglandin receptors [8] and endothelin receptors [9]. In addition, a large number of proteins, classically associated with receptor-mediated events at the cell surface, including heterotrimeric G proteins ([8,10,11]; reviewed in [12]), adenylyl cyclase isoforms [13,14], phospholipase A2[15], phospholipase Cβ [16], phospholipase D [17], RGS proteins (reviewed in [18]), β-arrestin1 [19,20], G protein-coupled receptor kinases [21–23], A kinase anchoring proteins (AKAPs) and PKA [24], among others, have been demonstrated to be trafficked to the nucleus and/or nuclear membrane.
In mammalian cardiomyocytes, three βAR subtypes are found which differ in their signalling partners, subcellular localization and desensitization profiles. β1ARs, localized to the sarcolemma and t-tubular network, plays a predominant role in regulating myocyte contractility. β2ARs, similarly distributed, play a more modest role in regulating inotropic and lusitropic responses. Both receptors signal through Gs and adenylyl cyclase with similar efficacy although the signals are compartmentalized differently possibly due to localization in distinct membrane microdomains and/or dual coupling of the β2AR to Gs and Gi (reviewed in [25–27]). β3ARs are also found in cardiomyocytes although their function remains ill-defined. Hence, βAR subtypes may serve unique functions and play non-redundant roles within the cardiomyocyte.
In the process of studying receptor trafficking and heterodimerization in mouse ventricular cardiomyocytes using recombinant adenoviruses, we noted a perinuclear distribution of overexpressed β1AR and β2AR [28]. We therefore wished to study receptor distribution in native myocytes and assess what function putative nuclear βAR might have.
2 Experimental procedures
2.1 Materials
Polyclonal rabbit anti-β1AR, β2AR, Gαs, Gαi1-3, adenylyl cyclases II, V/VI and polyclonal goat anti-β3AR were from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse anti-nucleoporin p62 (Nup-62) monoclonal antibodies (1:200) were from BD Transduction Laboratories. Appropriate TRITC-, CY5-, and HRP-conjugated secondary antibodies were from Jackson ImmunoResearch Laboratories (West Grove, PA). Chemiluminescence reagent Renaissance Plus was from Perkin-Elmer Life Sciences (Woodbridge, Ontario). Triton X-100, leupeptin, Protector RNAse inhibitor and PMSF were from Roche (Laval, Quebec). SDS-polyacrylamide gel electrophoresis reagents, nitrocellulose (0.22 μm) were from Bio-Rad (Mississauga, Ontario). Essential fatty acid-free BSA, CL 316243, PTX, isoproterenol, CGP 20712A and ICI 118,551 were from Sigma (Mississauga, Ontario). Xamoterol, procaterol and BRL 37344 were from Tocris (Ellisville, MO). Unless otherwise stated, all reagents were of analytical grade and were purchased from VWR Canlab (Ville Mont-Royal, Quebec) or Fisher Scientific (Mississauga, Ontario). [α32P]-UTP (specific activity 3000 Ci/mmol), [125I]-cyanopindolol (CYP) and [α32P]-ATP were from Perkin-Elmer.
2.2 Isolation of ventricular myocytes
Calcium-tolerant cardiomyocytes were isolated from adult rats [29] or mice [30] as described. These preparations provided 8–10 million cells/heart with 70–85% viability, as assessed by the presence of quiescent cells with rod-shaped morphology. The experimental protocol conformed with the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (NIH Publication No. 85-23, revised 1996).
2.3 Isolation of nuclei
Rat cardiac nuclei were isolated as described with modifications [31]. Briefly, rat hearts were pulverized under liquid nitrogen, resuspended in cold PBS and homogenized (Polytron, 8000 rpm; 2 × 10 s, Fraction 1). All subsequent steps were carried out on ice or at 5 °C. Homogenates were centrifuged for 15 min at 500 × g and supernatants, referred to as Fraction 2, diluted 1:1 with buffer A (10 mM K-HEPES (pH 7.9), 1.5 mM MgCl2, 10 mM KCl, 1 mM DTT, 25 μg/ml leupeptin, 0.2 mM Na3VO4), incubated 10 min on ice, and centrifuged for 15 min at 2000 × g. The resulting supernatant was discarded. The pellet, referred to as crude nuclei (Fraction 3) was resuspended in buffer B (0.3 M K-HEPES pH 7.9, 1.5 M KCl, 0.03 M MgCl2, 25 μg/ml leupeptin, 0.2 mM Na3VO4), incubated on ice for 10 min, and centrifuged for 15 min at 2000 × g. The pellet, an enriched nuclear fraction (Fraction 4), was resuspended in buffer C (20 mM Na-HEPES (pH 7.9), 25% (v/v) glycerol, 0.42 M NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.2 mM EGTA, 0.5 mM PMSF, 0.5 mM DTT, 25 μg/ml leupeptin, 0.2 mM Na3VO4) or 1X transcription buffer (see below) and either used fresh or aliquoted, snap-frozen using liquid nitrogen, and stored at − 80 °C.
2.4 Receptor quantification
Nuclei were prepared and washed as described above. Total βAR number was calculated using a saturating concentration (300 pM) of [125I]-cyanopindolol (CYP; Perkin Elmer) as the radioligand as described [32].
2.5 Measurement of adenylyl cyclase activity
Adenylyl cyclase activity was assayed in nuclear preparations as described [33] using 100 μg protein in a total volume of 50 μl. Enzyme activities were determined following a 15-min incubation in the presence of various receptor ligands, 100 μM forskolin, 10 mM NaF or vehicle at 37 °C. Data were calculated as pmol cAMP/min/mg protein, and analyzed by least squares regression using Prism 4.0cx (GraphPad Software).
2.6 Transcription initiation
Measurements of transcription initiation were as described [34]. Briefly, 10 μl of isolated nuclei were incubated at 30 °C for 30 min in 20 μl of 1 × transcription buffer (50 mM Tris pH 7.9, 0.15 M KCl, 1 mM MnCl2, 6 mM MgCl2, 1 mM ATP, 2 mM DTT, 1 U/μl RNAse inhibitor) and 10 μCi [α32P]-UTP (3000 Ci/mmol) in the absence of CTP and GTP to prevent chain elongation. After termination of reactions by digestion with DNase I, nuclei were lysed with 10 mM Tris–HCl pH 8.0, 10 mM EDTA and 1% SDS. Duplicate of 5 μl aliquots were dried onto Whatman GF/C glass fibre filters which were batch washed with 5% TCA, containing 20 mM sodium pyrophosphate and dried. 32P incorporation was determined by scintillation counting. DNA concentration was determined by spectrophotometry and incorporation expressed as dpm/μg DNA. Ligands were used as for activation of adenylyl cyclase. Where indicated, nuclei were pretreated with PTX (5 μg/ml) for 2 h.
2.7 Western blotting
Immunoblots were performed on the subcellular fractions described above. Protein concentrations were measured as described [35] using bovine γ-globulin as a standard [9]. We first demonstrated using HEK 293 cells, transfected or not with the three βAR subtypes that individual antibodies recognized cognate receptors specifically in both Western and immunocytochemical applications (data not shown). These antibodies have been used in other studies on rat ventricular cardiomyocytes under similar conditions [36,37]. Similarly, we performed control experiments for our other antibodies.
2.8 Confocal microscopy
The intracellular localization of βAR subtypes and downstream signalling partners was studied using scanning confocal microscopy (LSM 510 Carl Zeiss). Freshly isolated adult ventricular myocytes were plated on laminin-coated coverslips for 1 h (37 °C, 95% O2/5% CO2), fixed for 20 min in PBS (pH 7.4) containing 2% (w/v) paraformaldehyde, then washed thrice in PBS and blocked 1 h at room temperature in PBS containing 5% normal donkey serum (NDS) plus 0.2% (v/v) TX-100. Coverslips were rinsed thrice in PBS and incubated 1 h at 4 °C in PBS containing 10% NDS. Excess serum was removed and cells were incubated overnight at 4 °C with primary antibody diluted 1:100 in PBS containing 2% NDS. Coverslips were rinsed gently three times with PBS, drained, and incubated for 1 h at room temperature with a 1:500 (v/v) dilution of appropriate secondary antibodies (TRITC-conjugated anti-rabbit antibody, or ALEXA 488-conjugated anti-sheep antibody) in PBS containing 2% NDS. Coverslips were washed three times with PBS, drained, and mounted onto glass slides. For co-localization experiments, protocols were modified slightly. Freshly isolated mouse cardiomyocytes were plated on laminin-coated coverslips for 1 h (37 °C, 95% O2/5% CO2) and fixed for 20 min in 2% paraformaldehyde. Coverslips were rinsed three times in PBS and incubated 1 h at 4 °C in PBS containing 2% normal goat serum (NGS) and Triton 0.2% (v/v) when anti-β1AR (1:100) and β2AR (1:100) were used and 2% normal donkey serum (NDS) and Triton 0.2% (v/v) when β3AR (1:100) was used. ALEXA 555-conjugated anti-mouse antibody (1:500 in NGS) was used when murine cardiomyocytes were stained for Nup62. Fluorescence was visualized as serial 0.5-μm-thick optical sections in the z-axis plane of each cell. For each secondary antibody, control experiments were performed in the absence of primary antibody.
2.9 Statistical analysis
Data are presented as the mean±the standard error of the mean (S.E.). The significance of differences between groups was estimated by one-way ANOVA followed by Tukey's multiple comparison test (Prism 4.0cx, GraphPad Software). Differences were considered significant when p<0.05.
3 Results
3.1 Localization of β-adrenergic signalling machinery on nuclear membranes
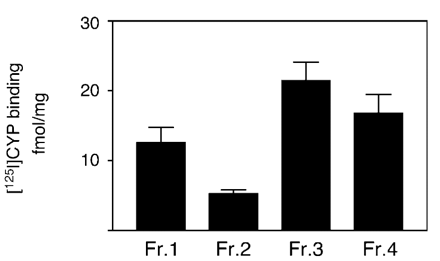
To determine whether βAR subtypes were trafficked to the nuclear membrane, we used a standard protocol for enrichment of nuclei. As shown in Fig. 1, binding of the βAR-specific ligand [125I]-CYP was detected in both crude (Fraction 3) and enriched (Fraction 4) nuclear preparations. The efficiency of fractionation is well characterized [9]. Fraction 3 and Fraction 4 are significantly enriched for the nuclear membrane marker, Nup62 [38] and have clearly lost both annexin II (a cell surface marker [39]), BiP (an ER chaperone [40]) and GM 130 (a Golgi resident protein [41]). The nuclear fraction is never totally pure but in going from Fraction 3 to Fraction 4, there is a significant enrichment for Nup62 (which was undetectable in Fractions 1 and 2) with concomitant depletion of markers for the cell surface (annexin II is reduced significantly in Fraction 4), ER (BiP is almost undetectable in Fraction 4), and Golgi (GM 130 is reduced significantly in Fraction 4). Initial experiments to determine the exact subtypes present in enriched nuclear fractions were performed using competition experiments with various subtype-selective ligands. These experiments could not clearly distinguish between the different subtypes in isolated nuclei. However, as described below, we used subtype-selective agonists to elicit specific responses in isolated nuclei.
125I-CYP binding in enriched nuclear fractions. Specific βAR binding (total minus non-specific as measured using 10 μM alprenolol) in various fractions associated with the nuclear enrichment procedure. Fractions are as described in Materials and methods. Data represent mean±S.E. of at least three separate experiments performed in triplicate.
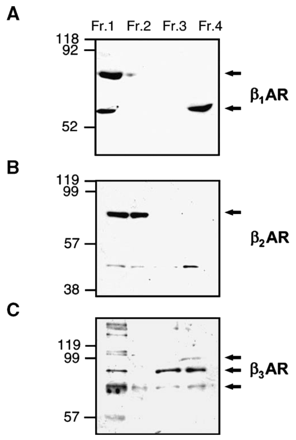
Similar results to those obtained in ligand binding studies were obtained using Western blots on the different crude and enriched nuclear fractions (Fig. 2). However, whereas all three subtypes could be detected in crude lysates (Fraction 1), only the β1AR and β3AR were detected in the enriched nuclear fraction (Fraction 4). A lower molecular weight protein (around 45 kDa) was revealed as a faint band by β2AR antiserum in Fractions 1–3; however this band was unrelated to the β2AR.
Detection of βAR isoforms in crude and enriched nuclei using Western blotting. Cell fractions were prepared as in Fig. 1. Lanes contained 100 μg protein from Fractions 1–4. Blots were probed using anti-β1AR (A), anti-β2AR (B) or anti-β3AR (C). Molecular weight markers are indicated on the left of the figure. Arrows indicate specific antibody signals previously described in the literature (see text for details). Data are representative of three independent experiments.
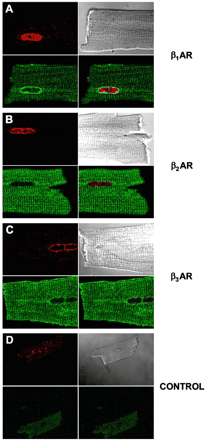
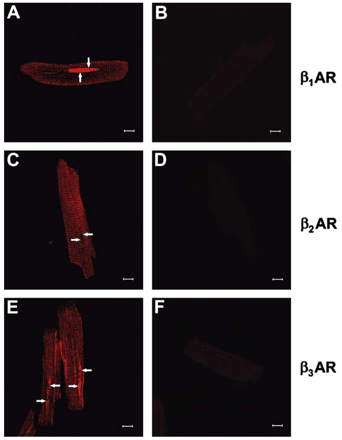
Next, confocal microscopy was used in order to further investigate the subcellular localization of βAR subtypes. Confocal image stacks were deconvolved to reduce the amount of out-of-focus haze from secondary antibodies [11]. Immunofluorescence for all three receptors was detected as punctate signals localized on the cell surface and in a striated pattern within myocytes (Fig. 3A, C, E). Although the t-tubular system is formed of continuous invaginations of the plasma membrane, β1AR and β2AR immunofluorescence was more intense on t-tubular membranes than surface membranes. Most interestingly, cell images of β1AR- or β3AR-decorated myocytes revealed an intense intracellular signal on the periphery of the nucleus (Fig. 3A) and in structures that appeared to extend outwards from its apex (Fig. 3E), indicating that they were localized in nuclear or perinuclear membranes. In contrast, no β2AR staining was detected on the nuclear membranes (arrows, Fig. 3A, C, E). No staining was observed in the absence of the primary antibody (Fig. 3B, D, F). Co-localization studies were also performed using mouse ventricular cardiomyocytes where relevant secondary antibodies were available and these confirmed our results using isolated rat cardiomyocytes. Nup-62 colocalized with β1AR and β3AR but not with β2AR (Fig. 4). Colocalization of all three subtypes was detected with caveolin-3 but not with the Golgi marker GM 130 (data not shown) confirming our results using subcellular fractionation.
Colocalization of βAR isoforms in adult mouse ventricular cardiomyocytes with a marker of the nuclear membrane. Panels A, B and C are representative confocal images showing the colocalization of β1AR, β2AR or β3AR, respectively with Nup-62, a component of the nuclear pore complex. Controls were performed in the absence of primary antibody (D). Data are representative of at least three independent experiments.
Detection of βAR isoforms in isolated adult rat ventricular cardiomyocytes using immunocytochemistry. Panels A, C and E are representative confocal images showing the distribution of β1AR (A), β2AR (C) or β3AR (E), respectively. Controls were performed in the absence of primary antibody (B, D and F). Scale bar is 10 μm. Arrows indicate nuclei. Data are representative of three independent experiments.
3.2 Functional effects of nuclear β-adrenergic receptors
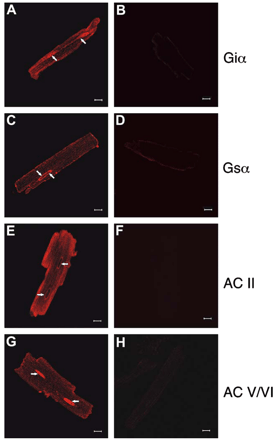
To assess potential function of the nuclear βAR receptors, immunofluorescence was employed to localize downstream effector molecules. Known downstream effectors, Gαi- and Gαs-subunits as well as adenylyl cyclases (AC) II and V/VI, were examined. Gαi immunofluorescence was apparent on t-tubules and surface membranes, including the intercalated disc region (Fig. 5A). In addition, punctate Gαi immunofluorescence was observed on the nuclear periphery and in intranuclear structures (arrow, Fig. 5A). Images from anti-Gαs-labeled cardiomyocytes revealed punctate immunofluorescence on plasma membranes, t-tubules and nuclear membranes, where staining was most intense (arrow, Fig. 5C). Thus, both G proteins known to couple to βAR subtypes in adult ventricular myocytes [25] were present on nuclear membranes and intranuclear structures. AC II antibodies decorated the cell surface, showed a striated intracellular pattern and labeled nuclei (Fig. 5E). AC II localization to t-tubules was mainly peripheral. AC V/VI immunofluorescence was observed at the surface membranes, t-tubules and was most intense in the intercalated disc regions and nuclei (Fig. 5G). Hence, the relevant βAR signalling machinery is present on nuclear membranes in cardiomyocytes.
Detection of βAR signalling partners in isolated adult rat left ventricular cardiomyocytes using immunocytochemistry. Panels A, C, E and G are representative confocal images showing the distribution of Giα (A), Gsα (C) ACII (E) or ACV/VI (G), respectively. Controls were performed in the absence of primary antibody (B, D, F and H). Scale bar is 10 μm. Arrows indicate nuclei. Data are representative of three independent experiments.
To more directly explore the functional consequences of nuclear localization of βAR, we first measured the ability of a non-selective agonist, isoproterenol, to stimulate adenylyl cyclase activity in isolated nuclei from rat hearts. We recognize that only 30–40% of the nuclei in the heart come from cardiomyocytes [42]. However, the mechanical procedure we used for nuclear isolation is much more efficient on whole tissues than isolated cells. As can be seen in Fig. 6A, there was a dose-dependent stimulation of adenylyl cyclase activity in enriched nuclear preparations demonstrating that the entire signalling system from receptor to Gs to effector is indeed functional. In the absence of ligand, basal enzyme activity was detected suggesting that the system may also be functional constitutively, i.e. even in the absence of the endogenous ligand (Fig. 6B). Direct activation of G protein with NaF or adenylyl cyclase with forskolin also led to an increase in the production of cAMP in isolated nuclei, confirming the functional presence of the signalling system downstream of the receptor (Fig. 6B). Interestingly, a β3AR-selective agonist CL 316243 had no effect on adenylyl cyclase activity, suggesting that the β1AR is primarily responsible for activation of this effector in nuclei.
Functional coupling of β1AR but not β3AR to adenylyl cyclase stimulation in enriched nuclear preparations. (A) Membranes isolated from enriched nuclear fractions were stimulated with increasing concentrations of isoproterenol (1 nM to 100 μM) and the production of [32P]-cAMP from [α32P]-ATP was assessed. (B) Stimulation of adenylyl cyclase activity in response to activation by 1 μM isoproterenol, the β3AR-selective agonist CL 316243 (1 μM), direct activation of G protein with 10 mM NaF or adenylyl cyclase with 100 μM forskolin. Data represent mean±S.E. of at least three separate experiments performed in triplicate.
To determine if there were consequences downstream of βAR activation particular to nuclear function, purified nuclei were incubated with [α32P]-UTP to measure initiation of RNA transcription in response to both non-selective and subtype-selective ligands. In addition to isoproterenol, subtype-selective ligands, xamoterol (β1AR), procaterol (β2AR) and BRL 37344 (β3AR) were used to stimulate transcription. Isoproterenol and BRL 37344, but not xamoterol (Fig. 7a) or procaterol (data not shown), could stimulate initiation of transcription. Since both Gs and Gi were detected on cardiomyocyte nuclear membrane, we next sought to determine the G protein coupling of each receptor. Nuclei were pretreated with PTX for 2 h prior to agonist stimulation in the presence of [α32P]-UTP. For both isoproterenol- and BRL 37344-stimulated nuclei, treatment with PTX abolished initiation of transcription demonstrating that the nuclear β3AR coupling to Gi is responsible for this activity. Further, basal activity was not effected by PTX treatment although the responses to both ligands became inhibitory. It is noteworthy to mention that stimulation of nuclear endothelin receptors induces a similar decrease in basal transcriptional activity (data not shown). This indicates that, under basal conditions, a balance exists between Gi-mediated stimulatory signals and inhibitory signals mediated by other G proteins. This notion is also supported by the observation that forskolin, a direct activator of adenylyl cyclase, tended toward an inhibitory effect (7525±2394 pmol cAMP/mg/min, basal versus 5637.2±1600 pmol cAMP/mg/min, 100 μM forskolin, n=6).
Functional coupling of β3AR but not β1AR to initiation of de novo transcription in enriched nuclear preparations. (A) Enriched nuclear fractions were stimulated with 1 μM isoproterenol, the β1AR-selective agonist xamoterol (1 μM) or the β3AR-selective agonist BRL 37344. [α32P]-UTP incorporation was measured in membranes either untreated or pretreated with 5 μg/ml PTX for 2 h to inhibit Gi-specific signalling. Significant differences (* for p<0.05 between control and ligand-stimulated) were determined by a paired t test for three or more experiments. (B) Enriched nuclear fractions were stimulated with 1 μM isoproterenol, with and without the β1AR-selective antagonist CGP 20712A (1 nM) or the β2AR-selective antagonist ICI 118,551 (0.5 nM). [α32P]-UTP incorporation was also measured in response to CGP 20712A or ICI 118,551 alone. Data represent mean±S.E. of at least three separate experiments performed in triplicate and are normalized to control. Significant differences (** and * for p<0.05 between vehicle and PTX-treated and control and ligand-stimulated, respectively) were determined by a paired t test for three or more experiments.
To further define the subtype selectivity for the transcriptional response and to overcome difficulties with the use of weak partial agonists such as xamoterol, we use either CGP 20712A or ICI 118,551 to block β1AR and β2AR, respectively. Curiously, we noted that isoproterenol-stimulated transcription initiation was inhibited in both cases (Fig. 7b). There was residual stimulation in the case of CGP 20712A. However, when we used these compounds, in the absence of agonist, CGP 20712A had no effect, consistent with its role as an antagonist. Surprisingly, ICI 118,551 had a strong inhibitory effect on the basal level of transcription initiation by itself. Further, we have shown that ICI 118,551 can compete for CYP binding (data not shown). These data suggest the possibility that levels of β2AR undetectable by immunological methods may be found in the nuclei as well.
Taken together, our data suggest that two effector systems are stimulated by βAR in isolated rat heart nuclei, one coupled to Gs which leads to an activation of adenylyl cyclase and another acting through Gi which leads to an increase in RNA transcription initiation.
4 Discussion
Our results demonstrate functional βAR receptors on the nuclear membrane. By immunological, ligand-binding and functional criteria, these seem to be predominantly β1AR and β3AR. However, it is also possible that the β2AR may be there but undetectable using available antibodies. These receptors were inhibited by ICI 118,551 even in the absence of agonist, i.e. ICI 118,551 is acting as an inverse agonist reducing constitutive receptor activity. CGP 20712 blocked the effects of isoproterenol as well. It has been demonstrated that CGP 20712 can antagonize β3AR [43] and this may explain the effects we have seen here. We have so far considered the effects of stimulation on nuclear βAR as if the receptors were monomeric or homodimeric entities. Alternatively, we [44] and others [45,46] have demonstrated that receptor heterodimers may comprise unique receptors with altered pharmacological properties, i.e. subtype-selective ligands may not be informative. Our results may thus be complicated by the possibility that the β1AR and β3AR may form heterodimers. Both β1AR and β2AR [47,48] and β2AR and β3AR [49] form heterodimeric receptors and it remains to be seen (although likely) if β1AR and β3AR do as well. Other potentially confounding and cell- or even organelle-specific factors might affect the pharmacology, signalling and/or trafficking of homodimeric or heterodimeric GPCRs to different subcellular destinations. As βAR signalling changes during development [50,51], and during the progression to heart failure [52], it is possible that trafficking itineraries on an absolute or relative basis may be altered as well.
β1AR and β3AR also seem to serve different functions in the nuclear membrane, as they are coupled to distinct G protein partners. β3AR stimulation, coupled to a PTX-sensitive G protein, likely Gi, which colocalizes with the receptor to the nuclear membrane, can initiate de novo transcription in isolated nuclei. The β3AR-mediated transcriptional activity is independent of adenylyl cyclase as stimulation with forskolin does not mimic these results. Our studies, being performed in native tissues, confirm that these results are not simply artifacts due to overexpression in heterologous expression systems and suggest a novel role for the β3AR in cardiac function. Also, we verified the existence of these receptors on the nucleus using multiple techniques, not only relying on antibodies but also demonstrating the presence of receptors and functional signalling pathways using ligand binding and by measuring two distinct agonist-mediated events. Further, as has been demonstrated for a number of other GPCRs, not only are the receptors themselves trafficked to the nuclear membrane but in many cases entire signalling pathways are also present.
A recent study that deorphanized GPR30 has demonstrated that this receptor is retained in the ER where it serves as a receptor for estrogen [53]. For other GPCRs such as the PAF or prostaglandin receptors, which are also activated by hydrophobic ligands which can cross the plasma membrane, it is quite possible to envision particular roles for these receptors in intracellular compartments. For receptors with peptidic ligands such as those for angiotensin, bradykinin or endothelin, it is intrinsically easier to imagine how these intracrine signalling pathways might be activated as well. This is because the ligands themselves may be released into intracellular compartments as part of their own independent trafficking itineraries following synthesis. In some cases, as for EGF receptors, a fragment of a cell surface receptor is trafficked to the nucleus after a proteolytic event [54,55]. This is unlikely to be the case here as Western blots of enriched nuclei indicate molecular weights for the β1AR and β3AR consistent with published values for full-length receptors [36,37]. Furthermore, no reduction in molecular weight was observed for the β1AR and β3AR in the nuclear fraction (Fraction 4) compared to total cell extract (Fraction 1). For adrenergic and other small ligand-activated receptors to have intracellular effects, one could consider an uptake mechanism. The classic reuptake system seen in neurons is unlikely to operate in cardiomyocytes. However, there are other non-selective uptake systems such as EMT (extraneuronal monoamine transporter, or OCT3) found in several tissues including heart ([56,57], reviewed in [58]) which could transport catecholamines to intracellular targets. One study using neonatal rat cardiomyocytes determined that extracellular [3H]-norepinephrine is taken up into these cells and >60% is distributed to nuclear fractions [59]. Further, these authors also demonstrate that nuclei isolated from these cells (purity confirmed by electron microscopy) possess approximately 60 fmol/mg βAR (roughly comparable to our values of 15–20 fmol/mg) as determined by ligand binding. It has also become clear in recent years that many GPCRs including βAR have constitutive, agonist-independent activity [60,61]. Depending on local expression levels in a given subcellular fraction, this constitutive activity could be quite important.
A growing number of GPCRs are trafficked to the nuclear or other endomembranes where they can activate signalling pathways that may be intrinsically different than those activated at the plasma membrane. The actual subcellular location of a given receptor may be critical for determining which signalling pathways get activated. Thus decisions about trafficking and formation of signalling complexes with distinct compositions may represent key events in determining the specificity of cellular communication.
Acknowledgements
TEH and BGA currently hold senior scholarships from the Fonds de la Recherche en Santé du Québec (FRSQ). BB was the recipient a studentship from the FRSQ. This work was supported by grants from NSERC, the Heart and Stroke Foundation of Québec and the Canadian Institutes of Health Research (CIHR) to TEH (MOP-36379) and BGA (MOP-64183) and from a CIHR group grant (FRN-12814). We thank Angelo Calderone for the discussion and critical advice. We also thank Céline Fiset and Marie-Andrée Lupien for assistance with isolation of mouse cardiomyocytes.
References
Author notes
Time for primary review 24 days
- cardiac myocytes
- isoproterenol
- signal transduction
- western blotting
- adenylate cyclase
- adult
- cell nucleus
- heart ventricle
- iodocyanopindolol
- ligands
- microscopy, confocal
- nuclear envelope
- procaterol
- beta-adrenergic receptors
- toxins
- xamoterol
- heart
- mice
- rats
- pertussis
- agonists
- signal pathway
- signal transduction pathways
- rna chemical synthesis



![Functional coupling of β1AR but not β3AR to adenylyl cyclase stimulation in enriched nuclear preparations. (A) Membranes isolated from enriched nuclear fractions were stimulated with increasing concentrations of isoproterenol (1 nM to 100 μM) and the production of [32P]-cAMP from [α32P]-ATP was assessed. (B) Stimulation of adenylyl cyclase activity in response to activation by 1 μM isoproterenol, the β3AR-selective agonist CL 316243 (1 μM), direct activation of G protein with 10 mM NaF or adenylyl cyclase with 100 μM forskolin. Data represent mean±S.E. of at least three separate experiments performed in triplicate.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/cardiovascres/71/1/10.1016/j.cardiores.2006.03.015/2/m_71-1-69-fig6.gif?Expires=1716451729&Signature=KcinDFUPzt2SqxT4dFZozvapZTEUsuDJajtredt35PQ1iNZQFTm4BXS79im6f9etFxxT15z6l68vIyM~QBAZKKx2cFrIom5SdylElwToInWy1xKOEzCprc4afa5HbaJeNZ4cgQNonZiYNiUP24DBqGlh9ldV~jeu1JTF2qq-614v5XHjWxuVw19eSWXbILwgZCLmjTNAalVXpg-3Nz-NG3948DV9EHJITiP4WqgAKS6XZ09flKDXtVb~iutoM699W7pJqbgGk-GoqtgNDALfsbDRaOjy3o3nBE1dwPag~2aylL-19yVaRKH8stRH0MHHj6gCIXtO-Q80dXt9IMToXQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Functional coupling of β3AR but not β1AR to initiation of de novo transcription in enriched nuclear preparations. (A) Enriched nuclear fractions were stimulated with 1 μM isoproterenol, the β1AR-selective agonist xamoterol (1 μM) or the β3AR-selective agonist BRL 37344. [α32P]-UTP incorporation was measured in membranes either untreated or pretreated with 5 μg/ml PTX for 2 h to inhibit Gi-specific signalling. Significant differences (* for p<0.05 between control and ligand-stimulated) were determined by a paired t test for three or more experiments. (B) Enriched nuclear fractions were stimulated with 1 μM isoproterenol, with and without the β1AR-selective antagonist CGP 20712A (1 nM) or the β2AR-selective antagonist ICI 118,551 (0.5 nM). [α32P]-UTP incorporation was also measured in response to CGP 20712A or ICI 118,551 alone. Data represent mean±S.E. of at least three separate experiments performed in triplicate and are normalized to control. Significant differences (** and * for p<0.05 between vehicle and PTX-treated and control and ligand-stimulated, respectively) were determined by a paired t test for three or more experiments.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/cardiovascres/71/1/10.1016/j.cardiores.2006.03.015/2/m_71-1-69-fig7.gif?Expires=1716451729&Signature=BF5FW0SWlAdVBoIUxo1snDenEXg0cjSvBei2n6Vfc7lWTXMTX9gBagZtqWpSSaCUnysYQKpgx8ReHoWlUvHx2XtIy6FQHEfYN-y2tIq~yD1JDsPprmxztTaVraiNCEdWvqLDu~ClJ~nJvz1h7cIw2HJQo~wCTfMYiTlzPgVbOYfyZ3Rz3LY7Y9QZQQVDpsp4TDZB409jiI6t2Be7Q6PxvS8VFD3jPmhkQJHkhYYrzc7AaHWRr2ozK9QTrRpt~P6mYn5ZQjJLF5xd1ooBHDKr0fqlfeAYG4Hg6y22JURy8FrbtwJHS170BqBqVL73xAbOM6VeZEg-ge0CvhV8NUovvQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
