-
PDF
- Split View
-
Views
-
Cite
Cite
Edward J. Vigmond, The electrophysiological basis of MAP recordings, Cardiovascular Research, Volume 68, Issue 3, December 2005, Pages 502–503, https://doi.org/10.1016/j.cardiores.2005.07.020
Close - Share Icon Share
Recently, differing views as to the genesis of monophasic action potential (MAP) recordings have surfaced [1–3]. Kondo et al. [1,3] claim that MAP recordings come about because core conductor theory dictates that the extracellular potential is a scaled version of the transmembrane voltage and that the contact electrode acts as a stationary ground. Franz [2] holds to the view that injury currents at the boundary of the contact electrode zone of influence lead to extracellular potential changes which reflect changes in transmembrane voltage. As a consequence of these divergent views, the importance of one electrode with respect to the other has come into question.
MAP electrodes are similar to potentials recorded with the sucrose gap technique [4]. That is, the technique functions as MAP recordings do, indirectly measuring transmembrane voltage by differencing extracellular potentials. Due to its relative simplicity, this technique warrants further consideration to determine if similar physics concepts are at work, which may shed light on the present issue. The sucrose gap technique separates two portions of an active cable with a perfusion of sucrose solution that serves to stop propagation of any activity across the gap due to the high resistivity of the sucrose region. The intracellular domains are still connected. By taking the difference in extracellular potentials on opposite sides of the sucrose gap, an approximation of the transmembrane voltage is obtained.
The sucrose gap works because the intracellular domains are well connected. Potential theory states that the voltage between two points is independent of the path traveled between the points. Thus, to find the voltage between the two extracellular points, we may follow a purely extracellular path or follow a path that crosses the membrane, travels intracellularly, and then crosses the membrane back into the extracellular space. By considering the latter path, it is clear how the extracellular potential follows the transmembrane voltage. The intracellular potential is nearly constant along the cable, and the transmembrane voltage is constant on the inactive side of the gap. On the active side of the gap, the extracellular potential is equal to intracellular potential plus the transmembrane voltage, which changes with time. Thus, taking the difference in extracellular potentials yields a waveform with the same shape as the transmembrane voltage on the active side but offset by the fixed transmembrane voltage of the inactive side.
The same principle may be at work in MAP recordings. Such a theory can satisfactorily account for all observations to date. To wit, we assume that by either pressure or addition of KCL, the transmembrane voltage in the region of the contact electrode is fixed to some value. We also assume that the intracellular domain is well connected and that its potential does not vary strongly spatially. Further, we assume that the extracellular potential must undergo a large change over a small distance at some region between the contact electrode and the indifferent electrode. But, without a sucrose gap, how is this achieved in the highly conductive medium? Again, by appealing to potential theory, we know that a closed surface of dipole sources will produce a discontinuity in potential as the surface is traversed. Here is where injury currents play their role. An extracellular dipole source is a small positive current monopole that is in close proximity to a negative current monopole. Thus, injury currents form local current loops flowing from the intracellular to extracellular domain and back at the edge of the region influenced by the contact electrode, and effectively function as dipole sources.
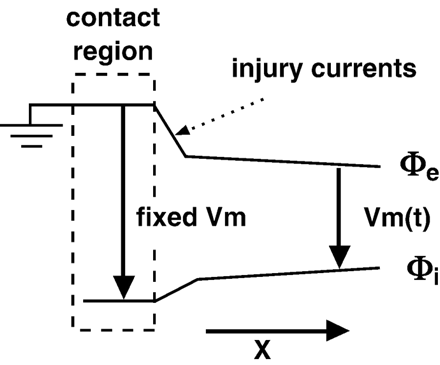
To understand why MAP recordings register an approximation of the transmembrane voltage, we start by considering the potential at the contact electrode as ground (see Fig. 1). The transmembrane voltage of the region under the electrode is also constant and thus, the intracellular potential is fixed. To reach the indifferent electrode, we follow a path that takes us intracellularly under the electrode and then across the membrane which has a time-varying voltage. Therefore, relative to the intracellular potential that is fixed with respect to ground, the extracellular potential will move with the transmembrane voltage. Of course, an ideal system has been described that will yield MAP recordings that are identical to transmembrane recordings.
Idealized spatial distribution of intracellular (φi) and extracellular (φe) potentials. Pressure from the contact electrode (or addition of KCl) fixes the transmembrane voltage (Vm) in the contact region. MAP recordings are produced by taking the difference of φe in the contact region and φe away from the contact region (indifferent electrode). Outside of the contact region, Vm is a function of time.
Many nonidealities exist in the system, which distorts the MAP recording. For example, the transmembrane potential under the contact electrode changes with time, the intracellular potential changes with distance as gap junctions are traversed, and the injury currents do not form a perfect dipole layer. Such discrepancies manifest as a negative notch immediately before the upstroke, a slower upstroke, reduced amplitude, and a slightly distorted wave shape. Nevertheless, the MAP recording has a very similar duration compared to the recorded transmembrane voltage.
In conclusion, both researchers are correct in their observations but one cannot talk of one electrode as being more important than the other. We are forever limited to measuring voltages that require two potentials. As Kondo et al. assert, the contact electrode can be considered a ground. As Franz points out, injury currents are also important to the genesis of MAP recordings. What each side in this debate is missing is the basic biophysical theory to successfully integrate all observations. In fact, the researchers were simply taking alternate paths to determine the potential – where Kondo et al. were following the intracellular core conductor route, Franz was following the extracellular route.
The electrophysiological basis for MAP recordings as outlined above was first proposed several years ago [5] but has remained largely unnoticed. Using a hybrid finite difference/boundary element approach, computer simulations showed that by assuming a fixed reversal potential and conductance for the volume under the electrode, MAP-like recordings could be generated. Plots of current density showed that there was indeed an intense inward current region surrounded by an outward current region (or vice versa) in the vicinity of the contact electrode. Predictions can be drawn from the theory and they can be tested. For example, intracellularly decoupling the contact electrode region from the rest of the tissue by closing gap junctions should eliminate MAP recordings. Tranquillo et al. later did a more detailed computer bidomain finite element study that further supports the proposed explanation [6].
This debate underscores the important place that modeling holds in our investigation of bioelectric phenomena. Experimental data needs a solid, comprehensive, theoretical framework from which conclusions and predictions can be made. Such a framework allows for both qualitative and quantitative predictions that can be tested in carefully designed experiments.
References