-
PDF
- Split View
-
Views
-
Cite
Cite
Coen van Solingen, Hetty C. de Boer, Roel Bijkerk, Matthieu Monge, Annemarie M. van Oeveren-Rietdijk, Leonard Seghers, Margreet R. de Vries, Eric P. van der Veer, Paul H.A. Quax, Ton J. Rabelink, Anton Jan van Zonneveld, MicroRNA-126 modulates endothelial SDF-1 expression and mobilization of Sca-1+/Lin− progenitor cells in ischaemia, Cardiovascular Research, Volume 92, Issue 3, 1 December 2011, Pages 449–455, https://doi.org/10.1093/cvr/cvr227
Close - Share Icon Share
Abstract
MicroRNA-126 (miR-126), which is enriched in endothelial cells, plays a role in angiogenesis. Based on the seed sequence, miR-126 can also be predicted to regulate vasculogenesis by modulating the endothelial expression of stromal cell-derived factor-1 (SDF-1).
Using miR-reporter constructs, we first validated that miR-126 inhibits SDF-1 expression in endothelial cells in vitro. Next, we investigated the potential relevance of this observation with respect to the mobilization of progenitor cells. For this, we studied the migration of human CD34+ progenitor cells towards chemotactic factors present in endothelial cell-conditioned medium. Antagomir-induced silencing of miR-126 elevated SDF-1 expression by human umbilical vein endothelial cells and enhanced migration of the CD34+ cells. In a murine model of hind limb ischaemia, a striking increase in the number of circulating Sca-1+/Lin− progenitor cells in antagomir-126-treated mice was observed when compared with scramblemir-treated controls. Immunohistochemical staining of capillaries in the post-ischaemic gastrocnemius muscle of miR-126-silenced mice revealed elevated SDF-1 expressing CD31-positive capillaries, whereas a mobilizing effect of miR-126 inhibition was not detected in healthy control animals.
miR-126 can regulate the expression of SDF-1 in endothelial cells. In the context of an ischaemic event, systemic silencing of miR-126 leads to the mobilization of Sca-1+/Lin− progenitor cells into the peripheral circulation, potentially in response to elevated SDF-1 expression by endothelial cells present in the ischaemic tissue.
1. Introduction
Integrity of the vascular endothelium is central to vascular homeostasis and is determined by the balance between endothelial injury and repair.1 In the context of adverse haemodynamic and metabolic risk factors, endothelial cells (ECs) are damaged and constantly replaced by the proliferation of adjacent mature ECs. In the rat aorta, ECs in areas resistant to atherosclerosis have a low rate of cellular replication and have been estimated to have a lifespan of ∼12 months. In contrast, EC turnover in lesion-prone sites is accelerated to weeks or less as animals age.2 To maintain the integrity of the endothelium throughout life, ECs in the arterial wall would therefore have to replicate well over a thousand times. However, primary EC cultures already become senescent after a limited number of passages due to a progressive shortening of telomeres that takes place upon each cell division. Moreover, telomere erosion is accelerated by chronic exposure of ECs to oxidative stress.3 Consequently, it has been suggested that mature ECs are insufficiently capable of repairing the chronically damaged endothelium throughout life. Support for this notion has been provided by several studies identifying a significant contribution by endothelial progenitor cells as an alternative cellular source for re-endothelialization of the damaged artery wall.4–6
Stromal cell-derived factor-1 (SDF-1 or CXCL12) has been demonstrated to facilitate the homing of progenitor cells from the peripheral circulation to sites of vascular injury or tissue ischaemia. SDF-1 can be actively secreted by injured ECs7 or activated platelets,8 leading to the homing of bone marrow-mobilized progenitor cells to the site of injury.9,10 The beneficial role of SDF-1 in recruiting bone marrow cells to ischaemic tissue has been established in several animal models to improve recovery after an ischaemic event.7,11–13 Therefore, SDF-1 is believed to play a central role in regulating both vascular integrity and homeostasis, implicating that its expression should be tightly controlled. Indeed SDF-1 expression is reported to be regulated at multiple levels including transcription and post-translation.7
At the post-transcriptional level, SDF-1 expression could be regulated by microRNAs (miRNAs). MiRNAs constitute a class of highly conserved non-coding RNAs that control gene expression by inhibiting the translation of mRNA.14 The ability of miRNAs to regulate multiple targets provides a means for the coordinated control of gene expression and make these molecules especially attractive candidates for regulating cell type-specific differentiation and modulating cell function.15 In recent years, miRNA expression-profiles of ECs have been analysed in detail and recent studies demonstrated both pro-angiogenic16–19 as well as anti-angiogenic functions for endothelial miRNAs.20,21
In silico analyses (http://www.microrna.org) for miRNAs targeting the 3′ untranslated region (3′ UTR) of the SDF-1 mRNA identified miR-126 as a potential post-transcriptional regulator of SDF-1 expression. MiR-126 is highly enriched in ECs and has been demonstrated to regulate (ischaemia-induced) angiogenesis by blocking the expression of SPRED-1 and PI3KR2.22–24 Here, we validated the inhibitory actions of miR-126 on expression and function of SDF-1 in ECs in vitro. Furthermore, we demonstrate that antagomir-induced silencing of miR-126 prior to acute ischaemia leads to the augmentation of SDF-1 levels in both the circulation and ischaemic tissue. This is associated with increased mobilization of bone marrow-derived progenitor cells in vivo. Our data demonstrate that endothelial miR-126 can regulate both features of angiogenesis as well as vasculogenesis and suggest a role for miR-126 in the maintenance of endothelial homeostasis.
2. Methods
2.1 Cells and cell culture
Immortalized human umbilical vein ECs (EC-RF24)25 were cultured in M199 medium (Gibco, Breda, the Netherlands) supplemented with penicillin/streptomycin (Gibco/Invitrogen), 20% foetal calf serum (FCS, Bio Whittaker/Cambrex, Verviers, Belgium), 10 IU/mL heparin (Leo Pharma, Breda, the Netherlands), 2.5% HEPES buffer (Gibco), and 12.5 μg/mL EC growth supplement (Sigma-Aldrich, St Louis, MO, USA). Human umbilical vein endothelial cells (HUVECs) were isolated, cultured and characterized as described previously.26 In short, ECs were isolated from freshly obtained human umbilical cords by trypsin/EDTA digestion of the interior of the umbilical vein. The cells were cultured in M199 medium supplemented with penicillin/streptomycin, 20% FCS, 10 IU/mL heparin, and 5% bovine pituitary extract (Gibco). CD34+ cells were isolated from umbilical cord blood using Ficoll (Amersham, ‘s-Hertogenbosch, the Netherlands) density gradient centrifugation and positive selection using CD34-MACS microbeads (Miltenyi Biotech, Bergish Gladbach, Germany). Umbilical cord blood and umbilical cords were collected non-identifiable and with informed consent. Since the human tissues were unidentifiable, approval from the university ethics review board was not necessary.
2.2 SDF-1 3′ UTR reporter assays
Synthetic, double-stranded oligonucleotides spanning a 60 bp region of the murine 3′ UTR of SDF-1 mRNA containing the putative miR-126 binding site with (pSDFmm) or without (pSDF) a C to G mismatch at position 3 of the seed-sequence were cloned into the pMIR-reportTM Expression Reporter Vector System (Applied Biosystems, Amsterdam, the Netherlands; see Supplementary material online for oligonucleotide sequences). All plasmids were sequenced to confirm their structure and exclude cloning artefacts. A renilla luciferase expression plasmid (pRL-SV40, Promega, Leiden, the Netherlands) and a plasmid containing a single, perfect match miR-126 binding site served as controls.23
2.3 Design of antagomirs
Antagomir-126 and a control scramblemir (scr) (Dharmacon RNA technologies, Lafayette, CO, USA) were synthesized as previously described.23
2.4 Luciferase assay
Antagomir-126 or control scr (5 μg/mL) was added to a near confluent layer of fibronectin-adherent EC-RF24 cells. Twenty-four hours after antagomir treatment, the EC-RF24 cells were detached by trypsinization and resuspended in 500 μL serum-free Optimem culture medium (Gibco) and 1.5 μg-specific pMIR-report and 150 ng pRL-SV40 were added. The cell suspension was chilled for 10 min at 4°C and electroporated using the Gene Pulser II (Bio-Rad Laboratories, Veenendaal, the Netherlands). After 10 min recovery time at room temperature, 1.5 × 105 cells were plated in a 24-wells plate coated with fibronectin in triplicate. After 24 h, the firefly-luciferase and renilla-luciferase signals were measured using a Dual-Luciferase® Assay Reporter System (Promega) in a Lumat LB9507 luminometer (EG&G Berthold, Bundoora, Australia).
2.5 Western blot
HUVECs were incubated for 48 h with 5 μg/mL antagomir-126 or scr. Culture medium was aspirated and cells were washed two times with PBS, cellular lysates were harvested using lysis buffer [50 mM Tris–HCl (pH = 7.5), 150 mM NaCl, 1% SDS, 0.5% sodiumdeoxycholate and 0.5% Triton X-100 (Sigma-Aldrich)]. Protein levels for SDF-1 and β-actin were assessed by western blot analysis with chemiluminescence detection. Equal amounts of protein were resolved on 15% SDS-polyacrylamide gels and transferred to PVDF membranes (Millipore, Billerica, MA, USA). SDF-1 was detected using a polyclonal rabbit antibody against human SDF-1 (0.5 μg/mL, Abcam, Cambridge, UK) and for β-actin a polyclonal rabbit antibody against human β-actin (0.1 μg/mL, Abcam) was used. Horseradish peroxidase-conjugated goat-anti-rabbit IgG (0.05 μg/mL, DakoCytomation, Enschede, the Netherlands) was used as secondary antibody. Bound fragments were detected with chemiluminescent reagents (Supersignal West Dura Extended Duration Substrate, Thermoscientific) and exposed on Hyperfilm ECL (Amersham). Quantitative analysis of the SDF-1 band intensity on western blot was performed using imageJ software and normalized for β-actin. The ratio of the SDF-1 band intensity over the β-actin band intensity of the antagomir-126-treated HUVEC was arbitrarily set at 100%.
2.6 Migration assay
HUVECs were incubated in serum-free, cell-specific medium with 0.1% insulin-transferrin-sodium selenite media supplement (ITS, Sigma-Aldrich) containing antagomir-126 or scr (5 μg/mL) at 37°C. After 48 h, cell supernatants were harvested and placed into the lower compartment of a transwell system with a pore size of 5 μm (Corning B.V. Life Sciences, Amsterdam, the Netherlands). Human CD34+ cells were added to the upper chamber and migration was followed over a period of 4 h at 37°C. As a positive control, 50 ng/mL recombinant human SDF-1(R&D Systems) was added in the lower compartment. The SDF-1-receptor, CXCR4, was neutralized by incubation of CD34+ cells with a blocking antibody against CXCR4 (8 μg/mL, R&D Systems) for 30 min at 4°C. After 4 h, the cell suspensions in the lower compartment were harvested, spun down and incubated for 30 min at 4°C in FACS buffer [PBS +1% bovine serum albumin (BSA, Sigma-Aldrich)] with directly conjugated antibodies against human CD34 (PerCP-Cy5, BD Biosciences, Breda, the Netherlands) and human CD133 (APC, Miltenyi Biotec). A fixed number of CD34+ cells was fluorescently labelled by incubation with calcein-AM (R&D Systems, Minneapolis, MN, USA) and added prior to an FACS analysis.
2.7 Hind limb ischaemia model
All animal experimental protocols were approved by the animal welfare committee of the Netherlands Organization for Applied Scientific Research (TNO, Leiden, the Netherlands) and conform the Directive 2010/63/EU of the European Parliament.
One day prior to surgery, C57BL/6 (wild-type) WT mice (n = 6 per group, age = 10 weeks, Charles River, Maastricht, the Netherlands) were injected intravenously (200 μL) with antagomir-126 (1.0 mg/animal) or scr (1.0 mg/animal). Before surgery, mice were anaesthetized intraperitoneally with a combination of Midazolam (5 mg/kg, Roche Diagnostics, Almere, the Netherlands), Medetomidine (0.5 mg/kg, Orion Corporation, Turku, Finland), and Fentanyl (0.05 mg/kg, Janssen Pharmaceutica, Tilburg, the Netherlands). Ischaemia of the left hind limb was induced by electrocoagulation of the left common femoral artery, proximal to the bifurcation of superficial and deep femoral artery, as described.27 To sacrifice the mice, blood was withdrawn for the FACS analysis (EDTA-anti-coagulated) by the heart puncture and the tibia and femur of both legs were kept for isolation of bone marrow cells. Additionally, the gastrocnemius muscle of both hind limbs was dissected and placed in 4% formaldehyde overnight. After paraffin embedding, 4-μm thick serial cross-sections were made for immunohistochemical analysis.
2.8 Whole blood analysis
Whole blood was collected by incision of the tail vein or heart puncture and analysed by semi-automatic haematology analyzer F-820 (Sysmex; Sysmex Corporation, Etten-Leur, the Netherlands), FACS analysis or ELISA. Haematological values obtained were white blood cell counts (WBC, n× 106/mL), red blood cell counts (RBC, n× 109/mL), platelets (PLT, n × 106/mL), haematocrit (HCT, %/%), and haemoglobin (HGB, mmol/L). For the FACS analysis, we incubated 50 μL of whole blood for 60 min at 4°C with directly conjugated antibodies directed against Sca-1 (FITC, BD-Biosciences), Flk-1 (PE, BD-Pharmingen, San Diego, CA, USA) and a cocktail against lineage-positive cells (APC, BD-Pharmingen). In a separate tube, 50 μL of whole blood was incubated with an appropriate cocktail of isotype controls, to identify the threshold for lineage-positivity. SDF-1 levels in serum were assessed with ELISA (R&D Systems).
2.9 Immunohistochemistry
Four micrometre thick sections of murine gastrocnemius muscle were re-hydrated and incubated with antigen-retrieval buffer (0.1% trypsin/EDTA in PBS). Next, sections were incubated with specific antibodies against murine SDF-1 (rabbit polyclonal IgG, Thermoscientific, Rockford, IL, USA) and murine CD31 (mouse monoclonal IgG2b, Santa Cruz, Heidelberg, Germany) followed by secondary antibodies against goat-anti-rabbit-IgG labelled with Alexa-488 or goat-anti-mouse IgG labelled with Alexa-568 (Molecular Probes). As a negative control, isotype-matched IgGs were used. Images were made using a confocal microscope (Carl Zeiss, Sliedrecht, the Netherlands).
2.10 FACS analysis
All samples obtained for the FACS analysis were either immediately analysed by flow cytometry analysis (FACS LSRII, BD Biosciences) or were fixed in 1% paraformaldehyde and analysed within 24 h after preparation. Data were analysed using FACSDiVa software (BD Biosciences).
2.11 Statistical analysis
Results are expressed as mean ± standard error of the mean (SEM). Statistical analysis was performed using the Mann–Whitney t-test. P < 0.05 was considered statistically significant.
3. Results
3.1 MiR-126 affects the expression and function of SDF-1 in vitro
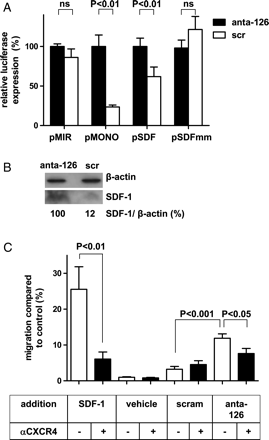
Using an on line miRNA target search tool (http://www.microrna.org) we identified a putative miR-126 binding site in the 3′ UTR of the SDF-1 mRNA. To assess its functionality, we cloned this binding site into the 3′ UTR of a luciferase reporter gene driven by a constitutive promoter (pSDF) and analysed luciferase expression and of a control plasmid in the human endothelial cell line EC-RF24. In these miR-126 expressing cells, luciferase expression driven from a reporter construct lacking a miRNA seed sequence (pMIR) was not affected when miR-126 was silenced using an antagomir approach (anta-126). In contrast, a reporter gene carrying a single perfect miR-126 binding site (pMONO) was fully active when miR-126 was silenced but its expression was strongly reduced in the presence of a control scr. These studies confirm the functionality of miR-126 in EC-RF24 cells. Likewise, the presence of the putative SDF-1 miR-126 binding site in the reporter construct led to a 40% reduction in luciferase-expression in miR-126 expressing cells (Figure 1A, scramble vs. antagomir-126, P < 0.01). A single mismatch in the seed sequence of the putative SDF-1 miR-126 binding site (C to G at position 3 of the seed sequence, pSDFmm) alleviated miR-126-dependent repression of reporter gene expression, confirming the presence and specificity of the miR-126 binding site in the 3′ UTR of the SDF-1 mRNA. To validate the regulatory effect of miR-126 on endogenous SDF-1 protein expression in ECs, HUVECs were treated with antagomir-126 or scr, after which SDF-1-protein expression was determined by western blot analysis. A marked increase in SDF-1 protein expression was observed in HUVEC treated with antagomir-126 when compared with scr (Figure 1B).
MiR-126 affects the expression and function of SDF-1 in vitro. (A) EC-RF24 were pre-incubated with scramblemir or antagomir-126 and transfected with pMIR as negative control, pMONO as positive control, pSDF with the sequence of the SDF-1 miR-126 binding site or a variant with a single mismatch in the seed sequence (pSDFmm). Experiments were performed in triplicate and data are expressed as mean values ± standard error of the mean. (B) HUVECs were incubated for 48 h with antagomir-126 or scramblemir and conditioned cell supernatants subjected to western blot analysis. Quantitative analysis of SDF-1 band intensity on western blot was performed using image-J and normalized for β-actin with the antagomir-126 band set at 100%. (C) HUVECs (n = 4) were treated with antagomir-126, scramblemir or vehicle and the conditioned media were used to study the chemotactic activity towards human hematopoietic CD34+ cells in vitro. Recombinant SDF-1 was used as positive control and the contribution of the SDF-1 receptor (CXCR4) was studied with the use of a function-blocking antibody (αCXCR4) (n = 2).
Next, we assessed the potential of miR-126 to regulate the extent by which EC-derived SDF-1 attracts progenitor cells. To this end, conditioned medium derived from HUVEC, incubated with antagomir-126 or scr, was applied to the lower compartment of a transwell-system and umbilical cord blood-derived CD34+ cells were placed in the upper compartment. As shown in Figure 1C, silencing of miR-126 in HUVEC-enhanced migration of CD34+ cells when compared with the scr-control (P < 0.001). Moreover, neutralization of CXCR4 expressed by the CD34+ cells with a function-blocking antibody (αCXCR4, 8 μg/mL) decreased cell migration towards the conditioned supernatant derived from antagomir-126-treated HUVEC back to the scramble control levels (P < 0.05). As a control, maximal cellular migration was observed using medium containing recombinant human SDF-1 (50 ng/mL), which was significantly reduced by αCXCR4 (Figure 1C, P < 0.01). These data suggest that suppression of miR-126 in ECs in vitro can augment expression of functional SDF-1, leading to elevated progenitor cell migration.
3.2 MiR-126 affects progenitor cell mobilization via SDF-1 in vivo
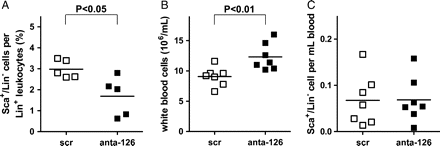
Endothelial miR-126 expression and functionality is conserved in murine EC, both in vivo23 and in vitro (Supplementary material online, Figures S3.1 and S3.2). To investigate the regulatory role of miR-126 on SDF-1-dependent progenitor cell mobilization in vivo, we applied single tail vein injections of antagomir-126 or the control scr into WT male C57Bl/6 mice (n = 6). Ten days after injection, we enumerated the number of circulating Sca-1-positive and lineage-negative (Sca-1+/Lin−) cells by the FACS analysis. Surprisingly, when we expressed the circulating Sca-1+/Lin− cell numbers as a percentage of Lin+ leucocytes, we observed a significant decrease in circulating Sca-1+/Lin− cells in the mice in which miR-126 had been silenced by antagomir-126 administration when compared with the scr-treated controls (Figure 2A, P < 0.05). This observation could be explained by the fact that antagomir-126-treated mice also displayed a significant increase in total WBC count (Figure 2B, P < 0.01). Such an increase is consistent with reports showing that increased SDF-1 levels in the circulation result in an elevation of WBCs.9 As such, when we expressed the number of circulating Sca-1+/Lin− cells per millilitre blood, the differences between antagomir-126 and scr-treated animals was lost, indicating that there is no systemic role for miR-126 in Sca-1+/Lin− progenitor cell mobilization in vivo (Figure 2C).
MiR-126 does not solely affect mobilization of Sca-1+/Lin− cells in vivo. (A) Ten days after injection of scramblemir or antagomir-126, Sca-1+/Lin− cells in whole blood were analysed by FACS, expressed as percentage of total Lin+ leucocytes. (B) Ten days after injection of scramblemir or antagomir-126, total white blood cell counts per millilitre was determined. (C) Ten days after scramblemir- or antagomir-126-injection Sca-1+/Lin− cells were analysed by FACS in whole blood expressed as total number in millilitre blood.
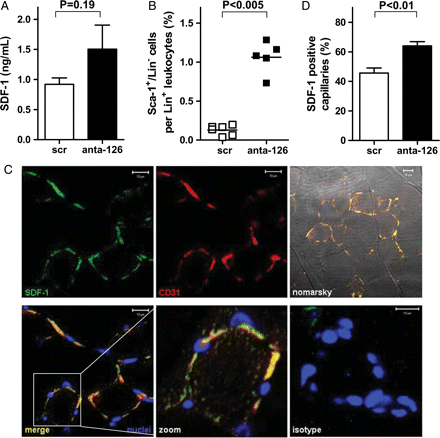
Since ischaemic ECs have been established to up regulate SDF-17 (and Supplementary material online, Figure S2.1). We investigated whether an ischaemic stimulus in combination with attenuation of miR-126 levels would modulate progenitor cell levels in mice. Therefore, we injected male WT mice (n = 6) with either antagomir-126 or scr and, the next day, this procedure was followed by the induction of unilateral hind limb ischaemia (HLI). Ten days after induction of HLI, the animals were sacrificed and serum levels of SDF-1 were assessed by ELISA. Although systemic SDF-1 levels were elevated in antagomir-126-treated mice when compared with controls, no significance was reached (Figure 3A, P = 0.19). Nevertheless, when we examined the in vivo mobilization of Sca-1+/Lin− cells to the circulation after ischaemia, we observed an eight-fold increase in Sca-1+/Lin− cells expressed as percentage of total Lin+ leucocytes in the blood of antagomir-126-treated animals when compared with scr-treated control animals (Figure 3B, P < 0.005). The absolute number of Sca-1+/Lin− cells per millilitre blood was raised from 0.24 ± 1.4 in the control group to 2.1 ± 1.4 in the antagomir-126-treated animals. As the observed effects suggested an ischaemia-dependent elevation of SDF-1, we next investigated whether the ischaemic gastrocnemius muscle could be a potential contributor to the elevated levels of circulating SDF-1. Therefore, we performed both detailed qualitative and quantitative immunohistochemical analysis of SDF-1 expression in relation to CD31-positive capillaries. Indeed, we observed a clear co-localization of SDF-1 and CD31 expression in sections of the ischaemic muscle (Figure 3C). Furthermore, the ischaemic muscle of antagomir-126-treated animals showed a higher percentage of double-positive capillaries (Figure 3D, P < 0.01) as opposed to scr-treated controls. These data imply that the mildly elevated levels of SDF-1 protein in the circulation upon antagomir-126-treatment may be a reflection of the locally enhanced expression of SDF-1 protein in the vessels of ischaemic tissue.
MiR-126 can alter SDF-1-expression and induce progenitor mobilization after ischaemic injury in vivo. (A) Ten days after HLI antagomir-126-treated mice showed non-significantly elevated levels of SDF-1 in the serum when compared with scramblemir-treated controls (n = 6). (B) Ten days after HLI and injection of scramblemir or antagomir-126, Sca-1+/Lin− cells were measured in whole blood, expressed as percentage of total Lin+ leucocytes (C), Immunohistochemical micrographs of immunohistochemistry of the gastrocnemius muscle after HLI showed colocalization of SDF-1 and CD31. SDF-1 was visualized with alexa-488 (green) and CD31 with alexa-568 (red). Nuclei were stained with DAPI and shown in blue, Nomarsky contrast images show muscle tissue. Scale bars represent 10 μm. (D) Quantification of microscopic images displayed that antagomir-126-treated mice have increased levels of SDF-1 positive capillaries as percentage of total number of capillaries (n = 6).
4. Discussion
A function for miR-126 in angiogenic processes in vascular maintenance and during development has been shown in a number of publications demonstrating that loss of miR-126, either in knockout models or mediated by treatment with antagomirs, leads to structural impairment of the vascular bed.22−24 In the current study, we provide evidence for a vasculogenic role for miR-126 in regulating the mobilization of endothelial progenitor cells via the release of chemokine SDF-1 from ischaemic ECs. In vitro, the increased secretion of SDF-1 upon silencing of miR-126 was sufficient to stimulate the migration of human CD34+ stem cells. In mice, however, systemic silencing with a single tail vein injection of antagomir-126 was not sufficient to raise the levels of circulating murine Sca-1+/Lin− progenitor cells. However, in combination with the ligation of the femoral artery, we demonstrated an increase in circulating Sca-1+/Lin− cells following miR-126 silencing, strongly suggesting that tissue ischaemia is needed to reveal the regulatory role of miR-126 in vivo.
Our data suggest that elevated numbers of circulating Sca-1+/Lin− cells in the antagomir-treated animals are the result of SDF-1-mediated mobilization of these cells following ischaemia. This is supported by the fact that SDF-1-protein expression is also up regulated in the ECs of the ischaemic tissue as well as in the peripheral circulation. Nevertheless, definitive proof of a causal role of miR-126 in SDF-1-dependent mobilization of progenitor cells would require an in vivo blockade in the SDF-1/CXCR4 axis. For this, inhibitors of the interaction of SDF-1 with CXCR4, such as AMD3100, could be administrated to antagomir-126-treated mice and used to alleviate the elevated progenitor cell mobilization following ischaemia. This approach is however complicated by the direct effects of these inhibitors on the egress of progenitor cells from the bone marrow which could potentially override the SDF-1 effects elicited by the endothelium in the ischaemic tissues in the periphery.28 Also, impaired progenitor homing to the ischaemic tissue or the spleen cannot be fully excluded.29
An interaction between miR-126 and SDF-1 has previously been shown to increase miR-126 uptake of EC-derived apoptotic bodies by ECs. This resulted in increased SDF-1 expression through inhibition of regulator of G-protein signalling 16.30 In contrast, our studies implicate that the abrogation of miR-126 is associated with increased expression of SDF-1, suggesting that miRNAs could serve as a biological rheostat, with the response magnitude of biological pathways being dependent on the context and source of the external stimulus.
Recently, it has been demonstrated that miRNAs are present in the circulation and that alterations in the profile of plasma or serum miRNAs can be associated with disease states.31,32 These early reports mainly displayed a link between circulating miRNAs and cancer, while subsequent studies have also revealed a clear association of circulating miRNAs with cardiovascular disease.33–35 Since endothelial injury is considered one of the hallmarks of patients at risk for cardiovascular disease and, upon injury, ECs can secrete miRNA-containing microvesicles,36–38 it is of interest to address the value of EC-derived circulating miRNAs as biomarkers for cardiovascular disease. Indeed, two recent clinical studies revealed a decrease in circulating miR-126 levels in patients with coronary artery disease (CAD)39 and diabetes mellitus 2 (DM2).40 It was suggested that lowered levels of miR-126 in DM2 patients might be explained by the fact that high glucose levels can lead to a decrease in the miR-126 content in the endothelial particles, while cellular miRNA levels remained unaltered.40 One may speculate that lowering of circulating miR-126 levels might be a specific signal of the injured endothelium to increase the expression levels of distinct miR-126 targets that are critical for the integrity of the endothelium.
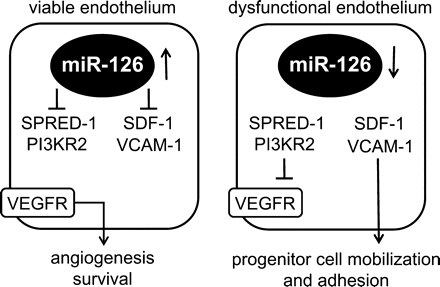
We suggest here that miR-126 functions as a regulator of endothelial homeostasis (Figure 4). In the healthy, viable endothelium, miR-126 is readily expressed and serves to down regulate SPRED-1 and PI3KR2, both of which are inhibitors of angiogenic and cell survival signals in response to vascular endothelial cell growth factor (VEGF).22,24 This condition favours the angiogenic response to injury, as SDF-1 expression is concomitantly repressed by miR-126. On the other hand, under conditions associated with EC dysfunction or senescence, a decrease in miR-126 levels would inhibit angiogenic and cell survival signals. Furthermore, this condition favours the expression of SDF-1 and vascular cell adhesion molecule 1 (VCAM-1)41 by the affected ECs and support re-endothelialization by a vasculogenic response through the recruitment and subsequent adhesion of vascular progenitor cells. As bone marrow-derived CD34+ cells were recently demonstrated to represent a more functional endothelial progentior cell (EPC) population than Sca-1+/Lin− progenitor cells, future studies may include these progenitor cell population as well.42
MiR-126 acts as a vasculogenic switch. In viable endothelium miR-126 inhibits the expression of SPRED-1 and PI3KR2 thereby facilitating VEGF-dependent angiogenesis. Furthermore, the expression of SDF-1 and VCAM-1 are inhibited. When miR-126 is lost, SPRED-1 and PI3KR2 are up regulated thereby blocking angiogenesis, at the same time SDF-1 and VCAM-1 levels are up regulated and this elevation subsequently leads to an increased mobilization and adhesion of bone marrow-derived progenitors.
Although speculative, our model is supported by the observed decreased levels of circulating miR-126 in DM2 and CAD patients39,40 and elevated levels of SDF-1 in patients with acute coronary syndrome (ACS).43 Future studies in patient cohorts will provide insight as to whether the down regulation of circulating miR-126 can be correlated with increased levels of SDF-1.
In conclusion, both in vitro and in vivo, miR-126 can regulate the expression of SDF-1 in ECs, which may lead to the mobilization of Sca-1+/Lin− progenitor cells into the peripheral circulation following conditions of acute ischaemia. As the endothelial miR-126 regulates both features of angiogenesis as well as vasculogenesis, a regulatory role for this miRNA in endothelial homeostasis is proposed.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: none declared.
Funding
C.v.S. is supported by the Netherlands Heart Foundation (grant number 2006B145). M.M. and R.B. are supported by the Dutch Kidney Foundation (grant number C08.2282 and C07.2227 respectively). E.v.d.V. is supported by the Translational Excellence in Regenerative Medicine (TeRM) Smart Mix Program of the Netherlands Ministry of Economic Affairs and the Netherlands Ministry of Education, Culture and Science. A.J.v.Z. is supported by a grant from the Genzyme Renal Innovations Program.